Effect of Mifepristone on Migration and Proliferation of Oral Cancer Cells
- PMID: 39201464
- PMCID: PMC11354386
- DOI: 10.3390/ijms25168777
Effect of Mifepristone on Migration and Proliferation of Oral Cancer Cells
Abstract
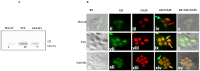
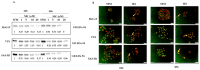
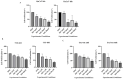
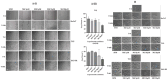
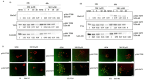
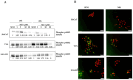
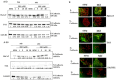
Glucocorticoid receptor (GR) overexpression has been linked to increased tumour aggressiveness and treatment resistance. GR antagonists have been shown to enhance treatment effectiveness. Emerging research has investigated mifepristone, a GR antagonist, as an anticancer agent with limited research in the context of oral cancer. This study investigated the effect of mifepristone at micromolar (µM) concentrations of 1, 5, 10 and 20 on the proliferation and migration of oral cancer cells, at 24 and 48 h. Scratch and scatter assays were utilised to assess cell migration, MTT assays were used to measure cell proliferation, Western blotting was used to investigate the expression of GR and the activation of underlying Phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) signalling pathways, and immunofluorescence (IF) was used to determine the localisation of proteins in HaCaT (immortalised human skin keratinocytes), TYS (oral adeno squamous cell carcinoma), and SAS-H1 cells (squamous cell carcinoma of human tongue). Mifepristone resulted in a dose-dependent reduction in the proliferation of HaCaT, TYS, and SAS-H1 cells. Mifepristone at a concentration of 20 µM effectively reduced collective migration and scattering of oral cancer cells, consistent with the suppression of the PI3K-Akt and MAPK signalling pathways, and reduced expression of N-Cadherin. An elongated cell morphology was, however, observed, which may be linked to the localisation pattern of E-Cadherin in response to mifepristone. Overall, this study found that a high concentration of mifepristone was effective in the suppression of migration and proliferation of oral cancer cells via the inhibition of PI3K-Akt and MAPK signalling pathways. Further investigation is needed to define its impact on epithelial-mesenchymal transition (EMT) markers.
Keywords: MAPK signalling pathway; PI3K/Akt signalling pathway; cell migration; glucocorticoid receptor; head and neck cancer; mifepristone; oral cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion?J Oral Pathol Med. 2020 Mar;49(3):227-234. doi: 10.1111/jop.12979. Epub 2019 Dec 22. J Oral Pathol Med. 2020. PMID: 31782565
-
Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways.Anticancer Res. 2013 May;33(5):1941-50. Anticancer Res. 2013. PMID: 23645742
-
Antiproliferative activity of an angular furanocoumarin-oroselol in human oral cancer cells is mediated via autophagy induction, inhibition of cell migration, invasion, and downregulation of PI3K/AKT signalling pathway.Acta Biochim Pol. 2022 Feb 28;69(1):85-89. doi: 10.18388/abp.2020_5713. Acta Biochim Pol. 2022. PMID: 35225439
-
Elevated Expression of Zinc Finger Protein 703 Promotes Cell Proliferation and Metastasis through PI3K/AKT/GSK-3β Signalling in Oral Squamous Cell Carcinoma.Cell Physiol Biochem. 2017;44(3):920-934. doi: 10.1159/000485360. Epub 2017 Nov 24. Cell Physiol Biochem. 2017. Retraction in: Cell Physiol Biochem. 2021;55(1):138. doi: 10.33594/000000347. PMID: 29176314 Retracted.
-
DKK3 knockdown confers negative effects on the malignant potency of head and neck squamous cell carcinoma cells via the PI3K/Akt and MAPK signaling pathways.Int J Oncol. 2019 Mar;54(3):1021-1032. doi: 10.3892/ijo.2018.4667. Epub 2018 Dec 14. Int J Oncol. 2019. PMID: 30569110
Cited by
-
Graph attention networks for predicting drug-gene association of glucocorticoid in oral squamous cell carcinoma: A comparison with GraphSAGE.PLoS One. 2025 Jul 3;20(7):e0327619. doi: 10.1371/journal.pone.0327619. eCollection 2025. PLoS One. 2025. PMID: 40608705 Free PMC article.
-
Dysregulated PI3K/AKT signaling in oral squamous cell carcinoma: The tumor microenvironment and epigenetic modifiers as key drivers.Oncol Res. 2025 Jul 18;33(8):1835-1860. doi: 10.32604/or.2025.064010. eCollection 2025. Oncol Res. 2025. PMID: 40746882 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

