Molecular Characteristics of the Malate Dehydrogenase (MDH) Gene Family in Spirometra mansoni (Cestoda: Diphyllobothriidea)
- PMID: 39201488
- PMCID: PMC11354392
- DOI: 10.3390/ijms25168802
Molecular Characteristics of the Malate Dehydrogenase (MDH) Gene Family in Spirometra mansoni (Cestoda: Diphyllobothriidea)
Abstract
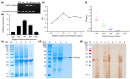
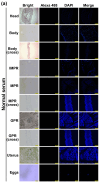
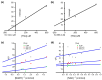
The plerocercoid larva of Spirometra mansoni can cause a parasitic zoonosis-sparganosis. Malate dehydrogenase (MDH) plays a very important role in the life activities of parasites. However, little is known about the MDH family in S. mansoni. We identified eight new MDH members in S. mansoni in this study. Clustering analysis divided SmMDHs into two groups and revealed patterns similar to the conserved motif organization. RT-qPCR suggested that five MDHs were highly expressed in the mature proglottid and that three MDHs were highly expressed in the gravid proglottid. Phylogenetic analysis revealed that SmMDHs contain both conserved family members and members in the process of further diversification. rSmMDH has an NAD binding domain, a dimer interface and a substrate binding domain. Natural SmMDH was immunolocalized in the tissues and follicles around the uterus in the mature or gravid proglottid and eggshells. The maximum forward and reverse reaction activities of rSmMDH were observed at pH 8.5 and 9.0, respectively. The optimum temperature for enzyme activity was 37 °C in the forward reaction and 40 °C in the reverse reaction. These results lay the foundation for studying the molecular functions and mechanisms of MDHs in S. mansoni and related taxa.
Keywords: Spirometra mansoni; gene expression; malate dehydrogenase; molecular characterization; tapeworm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wang R.J., Li W., Liu S.N., Wang S.Y., Jiang P., Wang Z.Q., Zhang X. Integrated transcriptomic and proteomic analyses of plerocercoid and adult Spirometra mansoni reveal potential important pathways in the development of the medical tapeworm. Parasit Vectors. 2023;16:316. doi: 10.1186/s13071-023-05941-8. - DOI - PMC - PubMed
-
- Musrati R.A., Kollárová M., Mernik N., Mikulásová D. Malate dehydrogenase: Distribution, function and properties. Gen. Physiol. Biophys. 1998;17:193–210. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

