Development of a Bmi1+ Cardiac Mouse Progenitor Immortalized Model to Unravel the Relationship with Its Protective Vascular Endothelial Niche
- PMID: 39201501
- PMCID: PMC11354400
- DOI: 10.3390/ijms25168815
Development of a Bmi1+ Cardiac Mouse Progenitor Immortalized Model to Unravel the Relationship with Its Protective Vascular Endothelial Niche
Abstract
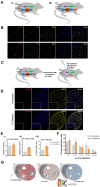
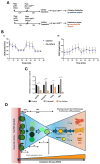
The adult mammalian heart has been demonstrated to be endowed with low but real turnover capacity, especially for cardiomyocytes, the key functional cell type. The source, however, of that turnover capacity remains controversial. In this regard, we have defined and characterized a resident multipotent cardiac mouse progenitor population, Bmi1+DR (for Bmi1+ Damage-Responsive cells). Bmi1+DR is one of the cell types with the lowest ROS (Reactive Oxygen Species) levels in the adult heart, being particularly characterized by their close relationship with cardiac vessels, most probably involved in the regulation of proliferation/maintenance of Bmi1+DR. This was proposed to work as their endothelial niche. Due to the scarcity of Bmi1+DR cells in the adult mouse heart, we have generated an immortalization/dis-immortalization model using Simian Vacuolating Virus 40-Large Antigen T (SV40-T) to facilitate their in vitro characterization. We have obtained a heterogeneous population of immortalized Bmi1+DR cells (Bmi1+DRIMM) that was validated attending to different criteria, also showing a comparable sensitivity to strong oxidative damage. Then, we concluded that the Bmi1-DRIMM population is an appropriate model for primary Bmi1+DR in vitro studies. The co-culture of Bmi1+DRIMM cells with endothelial cells protects them against oxidative damage, showing a moderate depletion in non-canonical autophagy and also contributing with a modest metabolic regulation.
Keywords: Bmi1; Bmi1-DR; SV40-T; autophagy; endothelial; heart; immortalization; metabolism; niche; oxidative damage; progenitor; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Age-related oxidative stress confines damage-responsive Bmi1+ cells to perivascular regions in the murine adult heart.Redox Biol. 2019 Apr;22:101156. doi: 10.1016/j.redox.2019.101156. Epub 2019 Mar 4. Redox Biol. 2019. PMID: 30851670 Free PMC article.
-
Redox-dependent BMI1 activity drives in vivo adult cardiac progenitor cell differentiation.Cell Death Differ. 2018 Mar;25(4):809-822. doi: 10.1038/s41418-017-0022-2. Epub 2018 Jan 11. Cell Death Differ. 2018. PMID: 29323265 Free PMC article.
-
Bmi1 (+) cardiac progenitor cells contribute to myocardial repair following acute injury.Stem Cell Res Ther. 2016 Jul 30;7(1):100. doi: 10.1186/s13287-016-0355-7. Stem Cell Res Ther. 2016. PMID: 27472922 Free PMC article.
-
The Vascular Niche for Adult Cardiac Progenitor Cells.Antioxidants (Basel). 2022 Apr 29;11(5):882. doi: 10.3390/antiox11050882. Antioxidants (Basel). 2022. PMID: 35624750 Free PMC article. Review.
-
Role of the polycomb group gene BMI1 in normal and leukemic hematopoietic stem and progenitor cells.Curr Opin Hematol. 2010 Jul;17(4):294-9. doi: 10.1097/MOH.0b013e328338c439. Curr Opin Hematol. 2010. PMID: 20308890 Review.
References
-
- Mollova M., Bersell K., Walsh S., Savla J., Das L.T., Park S.Y., Silberstein L.E., Dos Remedios C.G., Graham D., Colan S., et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

