In Situ Analyses of Placental Inflammatory Response to SARS-CoV-2 Infection in Cases of Mother-Fetus Vertical Transmission
- PMID: 39201511
- PMCID: PMC11355016
- DOI: 10.3390/ijms25168825
In Situ Analyses of Placental Inflammatory Response to SARS-CoV-2 Infection in Cases of Mother-Fetus Vertical Transmission
Abstract
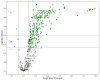
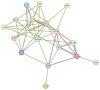
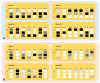
It has been shown that vertical transmission of the SARS-CoV-2 strain is relatively rare, and there is still limited information on the specific impact of maternal SARS-CoV-2 infection on vertical transmission. The current study focuses on a transcriptomics analysis aimed at examining differences in gene expression between placentas from mother-newborn pairs affected by COVID-19 and those from unaffected controls. Additionally, it investigates the in situ expression of molecules involved in placental inflammation. The Papa Giovanni XXIII Hospital in Bergamo, Italy, has recorded three instances of intrauterine transmission of SARS-CoV-2. The first two cases occurred early in the pandemic and involved pregnant women in their third trimester who were diagnosed with SARS-CoV-2. The third case involved an asymptomatic woman in her second trimester with a twin pregnancy, who unfortunately delivered two stillborn fetuses due to the premature rupture of membranes. Transcriptomic analysis revealed significant differences in gene expression between the placentae of COVID-19-affected mother/newborn pairs and two matched controls. The infected and control placentae were matched for gestational age. According to the Benjamani-Hochberg method, 305 genes met the criterion of an adjusted p-value of less than 0.05, and 219 genes met the criterion of less than 0.01. Up-regulated genes involved in cell signaling (e.g., CCL20, C3, MARCO) and immune response (e.g., LILRA3, CXCL10, CD48, CD86, IL1RN, IL-18R1) suggest their potential role in the inflammatory response to SARS-CoV-2. RNAscope® technology, coupled with image analysis, was utilized to quantify the surface area covered by SARS-CoV-2, ACE2, IL-1β, IL-6, IL-8, IL-10, and TNF-α on both the maternal and fetal sides of the placenta. A non-statistically significant gradient for SARS-CoV-2 was observed, with a higher surface coverage on the fetal side (2.42 ± 3.71%) compared to the maternal side (0.74 ± 1.19%) of the placenta. Although not statistically significant, the surface area covered by ACE2 mRNA was higher on the maternal side (0.02 ± 0.04%) compared to the fetal side (0.01 ± 0.01%) of the placenta. IL-6 and IL-8 were more prevalent on the fetal side (0.03 ± 0.04% and 0.06 ± 0.08%, respectively) compared to the maternal side (0.02 ± 0.01% and 0.02 ± 0.02%, respectively). The mean surface areas of IL-1β and IL-10 were found to be equal on both the fetal (0.04 ± 0.04% and 0.01 ± 0.01%, respectively) and maternal sides of the placenta (0.04 ± 0.05% and 0.01 ± 0.01%, respectively). The mean surface area of TNF-α was found to be equal on both the fetal and maternal sides of the placenta (0.02 ± 0.02% and 0.02 ± 0.02%, respectively). On the maternal side, ACE-2 and all examined interleukins, but not TNF-α, exhibited an inverse mRNA amount compared to SARS-CoV-2. On the fetal side, ACE-2, IL-6 and IL-8 were inversely correlated with SARS-CoV-2 (r = -0.3, r = -0.1 and r = -0.4, respectively), while IL-1β and IL-10 showed positive correlations (r = 0.9, p = 0.005 and r = 0.5, respectively). TNF-α exhibited a positive correlation with SARS-CoV-2 on both maternal (r = 0.4) and fetal sides (r = 0.9) of the placenta. Further research is needed to evaluate the correlation between cell signaling and immune response genes in the placenta and the vertical transmission of SARS-CoV-2. Nonetheless, the current study extends our comprehension of the molecular and immunological factors involved in SARS-CoV-2 placental infection underlying maternal-fetal transmission.
Keywords: COVID-19; SARS-CoV-2 vertical transmission; placenta; pregnancy; twins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Almulla N., Soltane R., Alasiri A., Kamal Allayeh A., Alqadi T., Alshehri F., Hamad Alrokban A., Zaghlool S.S., Zayan A.Z., Abdalla K.F., et al. Advancements in SARS-CoV-2 detection: Navigating the molecular landscape and diagnostic technologies. Heliyon. 2024;10:e29909. doi: 10.1016/j.heliyon.2024.e29909. - DOI - PMC - PubMed
-
- Patane L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020;2:100145. doi: 10.1016/j.ajogmf.2020.100145. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

