Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1
- PMID: 39201515
- PMCID: PMC11354443
- DOI: 10.3390/ijms25168829
Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1
Abstract
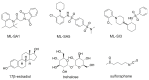
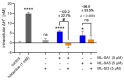
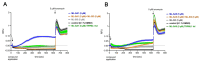
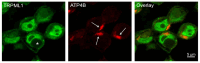
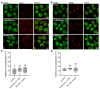
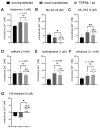
The lysosomal Ca2+ channel TRPML1 was found to be responsible for gastric acid secretion in murine gastric parietal cells by inducing the trafficking of H+/K+-ATPase containing tubulovesicles to the apical membrane. Therefore, we hypothesized a similar role of TRPML1 in regulating proton secretion in the immortalized human parietal cell line HGT-1. The primary focus was to investigate the involvement of TRPML1 in proton secretion using the known synthetic agonists ML-SA1 and ML-SA5 and the antagonist ML-SI3 and, furthermore, to identify food-derived compounds that target the channel. Proton secretion stimulated by ML-SA1 was reduced by 122.2 ± 22.7% by the antagonist ML-SI3. The steroid hormone 17β-estradiol, present in animal-derived foods, diminished the proton secretory effect of ML-SA1 by 63.4 ± 14.5%. We also demonstrated a reduction in the proton secretory effects of ML-SA1 and ML-SA5 on TRPML1 knock-down cells. The food-derived compounds sulforaphane and trehalose promoted proton secretion in HGT-1 cells but may act independently of TRPML1. Also, histamine- and caffeine-induced proton secretion were affected by neither the TRPML1 antagonist ML-SI3 nor the TRPML1 knock-down. In summary, the results obtained suggest that the activation of TRPML1 promotes proton secretion in HGT-1 cells, but the channel may not participate in canonical signaling pathways.
Keywords: Lamp1; MCOLN1; Mucolipin1; TRPML1; calcium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

