Function Analysis of a Maize Endo-1,4-β-xylanase Gene ZmHSL in Response to High-Temperature Stress
- PMID: 39201520
- PMCID: PMC11354693
- DOI: 10.3390/ijms25168834
Function Analysis of a Maize Endo-1,4-β-xylanase Gene ZmHSL in Response to High-Temperature Stress
Abstract
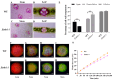
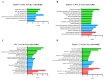
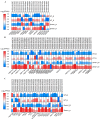
Rising temperature is a major threat to the normal growth and development of maize, resulting in low yield production and quality. The mechanism of maize in response to heat stress remains uncertain. In this study, a maize mutant Zmhsl-1 (heat sensitive leaves) with wilting and curling leaves under high temperatures was identified from maize Zheng 58 (Z58) mutant lines generated by ethyl methanesulfonate (EMS) mutagenesis. The Zmhsl-1 plants were more sensitive to increased temperature than Z58 in the field during growth season. The Zmhsl-1 plants had lower plant height, lower yield, and lower content of photosynthetic pigments. A bulked segregant analysis coupled with whole-genome sequencing (BSA-seq) enabled the identification of the corresponding gene, named ZmHSL, which encodes an endo-β-1,4-xylanase from the GH10 family. The loss-of-function of ZmHSL resulted in reduced lignin content in Zmhsl-1 plants, leading to defects in water transport and more severe leaf wilting with the increase in temperature. RNA-seq analysis revealed that the differentially expressed genes identified between Z58 and Zmhsl-1 plants are mainly related to heat stress-responsive genes and unfolded protein response genes. All these data indicated that ZmHSL plays a key role in lignin synthesis, and its defective mutation causes changes in the cell wall structure and gene expression patterns, which impedes water transport and confers higher sensitivity to high-temperature stress.
Keywords: BSA-seq; RNA-seq; cell wall; endo-β-1,4-xylanase; heat stress; maize; water transport.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- El-Sappah A.H., Rather S.A., Wani S.H., Elrys A.S., Bilal M., Huang Q., Dar Z.A., Elashtokhy M.M.A., Soaud N., Koul M., et al. Heat stress-mediated constraints in maize (Zea mays) production: Challenges and solutions. Front. Plant Sci. 2022;13:879366. doi: 10.3389/fpls.2022.879366. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

