Obesity-Associated Colorectal Cancer
- PMID: 39201522
- PMCID: PMC11354800
- DOI: 10.3390/ijms25168836
Obesity-Associated Colorectal Cancer
Abstract
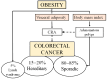
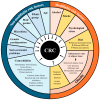
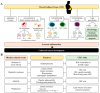
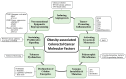
Colorectal cancer (CRC) affects approximately 2 million people worldwide. Obesity is the major risk factor for CRC. In addition, obesity contributes to a chronic inflammatory stage that enhances tumor progression through the secretion of proinflammatory cytokines. In addition to an increased inflammatory response, obesity-associated cancer presents accrued molecular factors related to cancer characteristics, such as genome instability, sustained cell proliferation, telomere dysfunctions, angiogenesis, and microbial alteration, among others. Despite the evidence accumulated over the last few years, the treatments for obesity-associated CRC do not differ from the CRC treatments in normal-weight individuals. In this review, we summarize the current knowledge on obesity-associated cancer, including its epidemiology, risk factors, molecular factors, and current treatments. Finally, we enumerate possible new therapeutic targets that may improve the conditions of obese CRC patients. Obesity is key for the development of CRC, and treatments resulting in the reversal of obesity should be considered as a strategy for improving antineoplastic CRC therapies.
Keywords: colorectal cancer; epidemiology; molecular factors; obesity; risk factors; targets; treatments.
Conflict of interest statement
G.K. has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys, and Vascage. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. G.K. is on the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds, and Reju-veron Life Sciences. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. Among these patents, one “Methods for weight reduction” (US11905330B1) is relevant to this study. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. G.K.’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. The funders had no role in the design of the study, in the writing of the manuscript, or in the decision to publish the results. The rest of the authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

