5β-Dihydrosteroids: Formation and Properties
- PMID: 39201544
- PMCID: PMC11354470
- DOI: 10.3390/ijms25168857
5β-Dihydrosteroids: Formation and Properties
Abstract
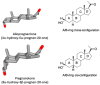
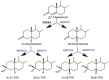
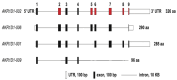
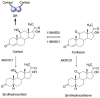
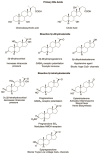
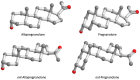
5β-Dihydrosteroids are produced by the reduction of Δ4-3-ketosteroids catalyzed by steroid 5β-reductase (AKR1D1). By analogy with steroid 5α-reductase, genetic deficiency exists in AKR1D1 which leads to errors in newborn metabolism and in this case to bile acid deficiency. Also, like the 5α-dihydrosteroids (e.g., 5α-dihydrotestosterone), the 5β-dihydrosteroids produced by AKR1D1 are not inactive but regulate ligand access to nuclear receptors, can act as ligands for nuclear and membrane-bound receptors, and regulate ion-channel opening. For example, 5β-reduction of cortisol and cortisone yields the corresponding 5β-dihydroglucocorticoids which are inactive on the glucocorticoid receptor (GR) and provides an additional mechanism of pre-receptor regulation of ligands for the GR in liver cells. By contrast, 5β-pregnanes can act as neuroactive steroids at the GABAA and NMDA receptors and at low-voltage-activated calcium channels, act as tocolytic agents, have analgesic activity and act as ligands for PXR, while bile acids act as ligands for FXR and thereby control cholesterol homeostasis. The 5β-androstanes also have potent vasodilatory properties and work through blockade of Ca2+ channels. Thus, a preference for 5β-dihydrosteroids to work at the membrane level exists via a variety of mechanisms. This article reviews the field and identifies gaps in knowledge to be addressed in future research.
Keywords: bile acids; farnesoid X receptor; neuroactive steroids; pregnane X receptor; smooth muscle relaxation; tocolysis.
Conflict of interest statement
Penning is a member of the Expert Panel Research Institute for Fragrance Materials, founder of Penzymes, LLC and has been a consultant for SAGE Therapeutics and Propella. Covey has equity in SAGE Therapeutics.
Figures


References
-
- Penning T.M., Burczynski M.E., Jez J.M., Hung C.F., Lin H.K., Ma H., Moore M., Palackal N., Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000;351:67–77. doi: 10.1042/bj3510067. - DOI - PMC - PubMed
-
- Steckelbroeck S., Jin Y., Gopishetty S., Oyesanmi B., Penning T.M. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J. Biol. Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

