The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes
- PMID: 39201563
- PMCID: PMC11355049
- DOI: 10.3390/ijms25168873
The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes
Abstract
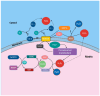
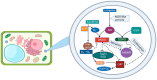
Nitric oxide (NO) has been firmly established as a key signaling molecule in plants, playing a significant role in regulating growth, development and stress responses. Given the imperative of sustainable agriculture and the urgent need to meet the escalating global demand for food, it is imperative to safeguard crop plants from the effects of climate fluctuations. Plants respond to environmental challenges by producing redox molecules, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), which regulate cellular, physiological, and molecular processes. Nitric oxide (NO) plays a crucial role in plant stress tolerance, acting as a signaling molecule or free radical. NO is involved in various developmental processes in plants through diverse mechanisms. Exogenous NO supplementation can alleviate the toxicity of abiotic stresses and enhance plant resistance. In this review we summarize the studies regarding the production of NO in peroxisomes, and how its molecule and its derived products, (ONOO-) and S-nitrosoglutathione (GSNO) affect ROS metabolism in peroxisomes. Peroxisomal antioxidant enzymes including catalase (CAT), are key targets of NO-mediated post-translational modification (PTM) highlighting the dynamic metabolism of ROS and RNS in peroxisomes.
Keywords: H2O2; S-nitrosoglutathione (GSNO); nitric oxide (NO); peroxynitrite (ONOO−), post-translational modification (PTM); reactive nitrogen species (RNS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Yadav S., Modi P., Dave A., Vijapura A., Patel D., Patel M. Effect of Abiotic Stress on Crops. In: Hasanuzzaman M., Fujita M., Teixeira Filho M.C.M., Nogueira T.A.R., Galindo F.S., editors. Sustainable Crop Production. IntechOpen; London, UK: 2020. - DOI
-
- Kumar V., Khare T. Differential growth and yield responses of salt-tolerant and susceptible rice cultivars to individual (Na+ and Cl−) and additive stress effects of NaCl. Acta Physiol. Plant. 2016;38:170. doi: 10.1007/s11738-016-2191-x. - DOI
-
- Barroso J.B., Valderrama R., Corpas F.J. Immunolocalization of S-nitrosoglutathione, S-nitrosoglutathione reductase and tyrosine nitration in pea leaf organelles. Acta Physiol. Plant. 2013;35:2635–2640. doi: 10.1007/s11738-013-1291-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

