Chikungunya and Mayaro Viruses Induce Chronic Skeletal Muscle Atrophy Triggered by Pro-Inflammatory and Oxidative Response
- PMID: 39201595
- PMCID: PMC11354814
- DOI: 10.3390/ijms25168909
Chikungunya and Mayaro Viruses Induce Chronic Skeletal Muscle Atrophy Triggered by Pro-Inflammatory and Oxidative Response
Abstract
Chikungunya (CHIKV) and Mayaro (MAYV) viruses are arthritogenic alphaviruses that promote an incapacitating and long-lasting inflammatory muscle-articular disease. Despite studies pointing out the importance of skeletal muscle (SkM) in viral pathogenesis, the long-term consequences on its physiology and the mechanism of persistence of symptoms are still poorly understood. Combining molecular, morphological, nuclear magnetic resonance imaging, and histological analysis, we conduct a temporal investigation of CHIKV and MAYV replication in a wild-type mice model, focusing on the impact on SkM composition, structure, and repair in the acute and late phases of infection. We found that viral replication and induced inflammation promote a rapid loss of muscle mass and reduction in fiber cross-sectional area by upregulation of muscle-specific E3 ubiquitin ligases MuRF1 and Atrogin-1 expression, both key regulators of SkM fibers atrophy. Despite a reduction in inflammation and clearance of infectious viral particles, SkM atrophy persists until 30 days post-infection. The genomic CHIKV and MAYV RNAs were still detected in SkM in the late phase, along with the upregulation of chemokines and anti-inflammatory cytokine expression. In agreement with the involvement of inflammatory mediators on induced atrophy, the neutralization of TNF and a reduction in oxidative stress using monomethyl fumarate, an agonist of Nrf2, decreases atrogen expression and atrophic fibers while increasing weight gain in treated mice. These data indicate that arthritogenic alphavirus infection could chronically impact body SkM composition and also harm repair machinery, contributing to a better understanding of mechanisms of arthritogenic alphavirus pathogenesis and with a description of potentially new targets of therapeutic intervention.
Keywords: arthritogenic alphavirus; chronic atrophy; inflammation; oxidative stress; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
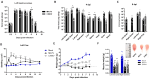


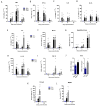



 block/inhibition;
block/inhibition;  positive regulation;
positive regulation;  open ended questions.
open ended questions.References
-
- Santiago F.W., Halsey E.S., Siles C., Vilcarromero S., Guevara C., Silvas J.A., Ramal C., Ampuero J.S., Aguilar P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015;9:e0004104. doi: 10.1371/journal.pntd.0004104. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

