The Comparison of Immunomodulatory Properties of Canine and Human Wharton Jelly-Derived Mesenchymal Stromal Cells
- PMID: 39201612
- PMCID: PMC11354339
- DOI: 10.3390/ijms25168926
The Comparison of Immunomodulatory Properties of Canine and Human Wharton Jelly-Derived Mesenchymal Stromal Cells
Abstract
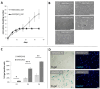
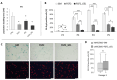
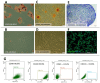
Although therapies based on mesenchymal stromal cells (MSCs) are being implemented in clinical settings, many aspects regarding these procedures require further optimization. Domestic dogs suffer from numerous immune-mediated diseases similar to those found in humans. This study aimed to assess the immunomodulatory activity of canine (c) Wharton jelly (WJ)-derived MSCs and refer them to human (h) MSCs isolated from the same tissue. Canine MSC(WJ)s appeared to be more prone to in vitro aging than their human counterparts. Both canine and human MSC(WJ)s significantly inhibited the activation as well as proliferation of CD4+ and CD8+ T cells. The treatment with IFNγ significantly upregulated indoleamine-2,3-dioxygenase 1 (IDO1) synthesis in human and canine MSC(WJ)s, and the addition of poly(I:C), TLR3 ligand, synergized this effect in cells from both species. Unstimulated human and canine MSC(WJ)s released TGFβ at the same level (p > 0.05). IFNγ significantly increased the secretion of TGFβ in cells from both species (p < 0.05); however, this response was significantly stronger in human cells than in canine cells. Although the properties of canine and human MSC(WJ)s differ in detail, cells from both species inhibit the proliferation of activated T cells to a very similar degree and respond to pro-inflammatory stimulation by enhancing their anti-inflammatory activity. These results suggest that testing MSC transplantation in naturally occurring immune-mediated diseases in dogs may have high translational value for human clinical trials.
Keywords: canine mesenchymal stromal cells; human mesenchymal stromal cells; immunomodulation; indoleamine 2,3-dioxygenase 1; transforming growth factor beta; umbilical cord.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



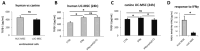
Similar articles
-
Extracellular Vesicles from Wharton's Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model.Stem Cells Dev. 2019 Feb 1;28(3):212-226. doi: 10.1089/scd.2018.0097. Epub 2019 Jan 14. Stem Cells Dev. 2019. PMID: 30412034
-
Wharton's Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: a Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro.Stem Cell Rev Rep. 2019 Dec;15(6):900-918. doi: 10.1007/s12015-019-09907-1. Stem Cell Rev Rep. 2019. PMID: 31741193
-
Human Wharton's Jelly-Derived Mesenchymal Stromal Cells Primed by Tumor Necrosis Factor-α and Interferon-γ Modulate the Innate and Adaptive Immune Cells of Type 1 Diabetic Patients.Front Immunol. 2021 Sep 28;12:732549. doi: 10.3389/fimmu.2021.732549. eCollection 2021. Front Immunol. 2021. PMID: 34650558 Free PMC article.
-
Characteristics and clinical applications of Wharton's jelly-derived mesenchymal stromal cells.Curr Res Transl Med. 2020 Jan;68(1):5-16. doi: 10.1016/j.retram.2019.09.001. Epub 2019 Sep 19. Curr Res Transl Med. 2020. PMID: 31543433 Review.
-
Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells.Cytotherapy. 2015 Jan;17(1):18-24. doi: 10.1016/j.jcyt.2014.08.009. Epub 2014 Oct 18. Cytotherapy. 2015. PMID: 25442786 Free PMC article. Review.
Cited by
-
Preventing MSC aging and enhancing immunomodulation: Novel strategies for cell-based therapies.Regen Ther. 2025 May 5;29:517-539. doi: 10.1016/j.reth.2025.04.014. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40453699 Free PMC article. Review.
References
-
- Cuerquis J., Romieu-Mourez R., François M., Routy J.-P., Young Y.K., Zhao J., Eliopoulos N. Human Mesenchymal Stromal Cells Transiently Increase Cytokine Production by Activated T Cells before Suppressing T-Cell Proliferation: Effect of Interferon-γ and Tumor Necrosis Factor-α Stimulation. Cytotherapy. 2014;16:191–202. doi: 10.1016/j.jcyt.2013.11.008. - DOI - PubMed
-
- Opitz C.A., Litzenburger U.M., Lutz C., Lanz T.V., Tritschler I., Köppel A., Tolosa E., Hoberg M., Anderl J., Aicher W.K., et al. Toll-Like Receptor Engagement Enhances the Immunosuppressive Properties of Human Bone Marrow-Derived Mesenchymal Stem Cells by Inducing Indoleamine-2,3-Dioxygenase-1 via Interferon-β and Protein Kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

