The Impact of Sciatic Nerve Injury on Extracellular Matrix of Lower Limb Muscle and Thoracolumbar Fascia: An Observational Study
- PMID: 39201630
- PMCID: PMC11354760
- DOI: 10.3390/ijms25168945
The Impact of Sciatic Nerve Injury on Extracellular Matrix of Lower Limb Muscle and Thoracolumbar Fascia: An Observational Study
Abstract
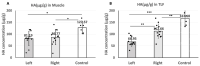
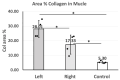
Peripheral nerve injury (PNI) is a complex clinical challenge resulting in functional disability. Neurological recovery does not always ensure functional recovery, as extracellular matrix (ECM) alterations affect muscle function. This study evaluates hyaluronan (HA) and collagen concentration in the gastrocnemius muscle and thoracolumbar fascia (TLF) in unilateral lower limb PNI rats to explore systemic ECM alterations following PNI and their impacts on functional recovery. Eighteen 8-week-old male Sprague-Dawley rats were divided into experimental (n = 12 left sciatic nerve injury) and control (n = 6) groups. After six weeks, motor function was evaluated. Muscle and TLF samples were analysed for HA and collagen distribution and concentrations. SFI and gait analysis confirmed a functional deficit in PNI rats 6 weeks after surgery. HA concentration in both sides of the muscles decreased by approximately one-third; both sides showed significantly higher collagen concentration than healthy rats (12.74 ± 4.83 µg/g), with the left (32.92 ± 11.34 µg/g) significantly higher than the right (20.15 ± 7.03 µg/g). PNI rats also showed significantly lower HA (left: 66.95 ± 20.08 µg/g; right: 112.66 ± 30.53 µg/g) and higher collagen (left: 115.89 ± 28.18 µg/g; right: 90.43 ± 20.83 µg/g) concentrations in both TLF samples compared to healthy rats (HA: 167.18 ± 31.13 µg/g; collagen: 47.51 ± 7.82 µg/g), with the left TLF more affected. Unilateral lower limb PNI induced HA reduction and collagen accumulation in both the lower limb muscles and the TLF, potentially exacerbating motor function impairment and increasing the risk of low back dysfunctions.
Keywords: collagen; extracellular matrix; hyaluronan; intramuscular connective tissue; peripheral nerve injury; thoracolumbar fascia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Aberg M., Ljungberg C., Edin E., Millqvist H., Nordh E., Theorin A., Terenghi G., Wiberg M. Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: A prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. J. Plast. Reconstr. Aesthet. Surg. 2009;62:1503–1509. doi: 10.1016/j.bjps.2008.06.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

