Different Strategies to Overcome Resistance to Proteasome Inhibitors-A Summary 20 Years after Their Introduction
- PMID: 39201634
- PMCID: PMC11354503
- DOI: 10.3390/ijms25168949
Different Strategies to Overcome Resistance to Proteasome Inhibitors-A Summary 20 Years after Their Introduction
Abstract
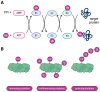
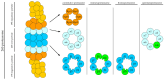
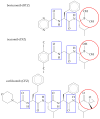
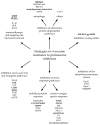
Proteasome inhibitors (PIs), bortezomib, carfilzomib, and ixazomib, are the first-line treatment for multiple myeloma (MM). They inhibit cytosolic protein degradation in cells, which leads to the accumulation of misfolded and malfunctioned proteins in the cytosol and endoplasmic reticulum, resulting in cell death. Despite being a breakthrough in MM therapy, malignant cells develop resistance to PIs via different mechanisms. Understanding these mechanisms drives research toward new anticancer agents to overcome PI resistance. In this review, we summarize the mechanism of action of PIs and how MM cells adapt to these drugs to develop resistance. Finally, we explore these mechanisms to present strategies to interfere with PI resistance. The strategies include new inhibitors of the ubiquitin-proteasome system, drug efflux inhibitors, autophagy disruption, targeting stress response mechanisms, affecting survival and cell cycle regulators, bone marrow microenvironment modulation, and immunotherapy. We list potential pharmacological targets examined in in vitro, in vivo, and clinical studies. Some of these strategies have already provided clinicians with new anti-MM medications, such as panobinostat and selinexor. We hope that further exploration of the subject will broaden the range of therapeutic options and improve patient outcomes.
Keywords: molecular medicine; proteasome inhibitors; treatment resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

