Long-Term, Sex-Specific Effects of GCRsim and Gamma Irradiation on the Brains, Hearts, and Kidneys of Mice with Alzheimer's Disease Mutations
- PMID: 39201636
- PMCID: PMC11355020
- DOI: 10.3390/ijms25168948
Long-Term, Sex-Specific Effects of GCRsim and Gamma Irradiation on the Brains, Hearts, and Kidneys of Mice with Alzheimer's Disease Mutations
Abstract
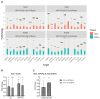
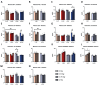
Understanding the hazards of space radiation is imperative as astronauts begin voyaging on missions with increasing distances from Earth's protective shield. Previous studies investigating the acute or long-term effects of specific ions comprising space radiation have revealed threats to organs generally considered radioresistant, like the brain, and have shown males to be more vulnerable than their female counterparts. However, astronauts will be exposed to a combination of ions that may result in additive effects differing from those of any one particle species. To better understand this nuance, we irradiated 4-month-old male and female, wild-type and Alzheimer's-like mice with 0, 0.5, or 0.75 Gy galactic cosmic ray simulation (GCRsim) or 0, 0.75, or 2 Gy gamma radiation (wild-type only). At 11 months, mice underwent brain and heart MRIs or behavioral tests, after which they were euthanized to assess amyloid-beta pathology, heart and kidney gene expression and fibrosis, and plasma cytokines. Although there were no changes in amyloid-beta pathology, we observed many differences in brain MRIs and behavior, including opposite effects of GCRsim on motor coordination in male and female transgenic mice. Additionally, several genes demonstrated persistent changes in the heart and kidney. Overall, we found sex- and genotype-specific, long-term effects of GCRsim and gamma radiation on the brain, heart, and kidney.
Keywords: Alzheimer’s disease; CNS; GCRsim; gamma; neurodegeneration; radiation; space radiation.
Conflict of interest statement
C.A.L. serves as a paid scientific advisor for Acumen Pharmaceuticals, ADvantage Therapeutics, Alnylam Pharmaceuticals, Apellis Pharmaceuticals, Biohaven Pharmaceuticals, Cyclo Therapeutics, Merck, MindImmune Therapeutics, Novo Nordisk, and Sanofi-Genzyme, and receives research funding from the National Institutes of Health (NIH), NASA, and the Cure Alzheimer’s Fund. All other authors have no conflicts to report.
Figures






References
-
- NASA. [(accessed on 28 May 2024)]; Available online: https://www.nasa.gov/missions/analog-field-testing/why-space-radiation-m...
-
- Hinshaw R.G., Schroeder M.K., Ciola J., Varma C., Colletti B., Liu B., Liu G.G., Shi Q., Williams J.P., O’Banion M.K., et al. High-Energy, Whole-Body Proton Irradiation Differentially Alters Long-Term Brain Pathology and Behavior Dependent on Sex and Alzheimer’s Disease Mutations. Int. J. Mol. Sci. 2023;24:3615. doi: 10.3390/ijms24043615. - DOI - PMC - PubMed
-
- Cherry J.D., Liu B., Frost J.L., Lemere C.A., Williams J.P., Olschowka J.A., O’Banion M.K. Galactic cosmic radiation leads to cognitive impairment and increased aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS ONE. 2012;7:e53275. doi: 10.1371/journal.pone.0053275. - DOI - PMC - PubMed
-
- Liu B., Hinshaw R.G., Le K.X., Park M.A., Wang S., Belanger A.P., Dubey S., Frost J.L., Shi Q., Holton P., et al. Space-like (56)Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer’s-like transgenic mice. Sci. Rep. 2019;9:12118. doi: 10.1038/s41598-019-48615-1. - DOI - PMC - PubMed
-
- Schroeder M.K., Liu B., Hinshaw R.G., Park M.A., Wang S., Dubey S., Liu G.G., Shi Q., Holton P., Reiser V., et al. Long-Term Sex- and Genotype-Specific Effects of (56)Fe Irradiation on Wild-Type and APPswe/PS1dE9 Transgenic Mice. Int. J. Mol. Sci. 2021;22:13305. doi: 10.3390/ijms222413305. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

