CD47 and IFT57 Are Colinear Genes That Are Highly Coexpressed in Most Cancers and Exhibit Parallel Cancer-Specific Correlations with Survival
- PMID: 39201643
- PMCID: PMC11354933
- DOI: 10.3390/ijms25168956
CD47 and IFT57 Are Colinear Genes That Are Highly Coexpressed in Most Cancers and Exhibit Parallel Cancer-Specific Correlations with Survival
Abstract
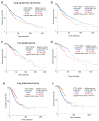
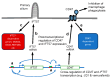
An association between high CD47 expression and poor cancer survival has been attributed to its function on malignant cells to inhibit phagocytic clearance. However, CD47 mRNA expression in some cancers lacks correlation or correlates with improved survival. IFT57 encodes an essential primary cilium component and is colinear with CD47 across amniote genomes, suggesting coregulation of these genes. Analysis of The Cancer Genome Atlas datasets identified IFT57 as a top coexpressed gene with CD47 among 1156 human cancer cell lines and in most tumor types. The primary cilium also regulates cancer pathogenesis, and correlations between IFT57 mRNA and survival paralleled those for CD47 in thyroid and lung carcinomas, melanoma, and glioma. CD47 ranked first for coexpression with IFT57 mRNA in papillary thyroid carcinomas, and higher expression of both genes correlated with significantly improved overall survival. CD47 and IFT57 mRNAs were coordinately regulated in thyroid carcinoma cell lines. Transcriptome analysis following knockdown of CD47 or IFT57 in thyroid carcinoma cells identified the cytoskeletal regulator CRACD as a specific target of IFT57. CRACD mRNA expression inversely correlated with IFT57 mRNA and with survival in low-grade gliomas, lung adenocarcinomas, and papillary thyroid carcinomas, suggesting that IFT57 rather than CD47 regulates survival in these cancers.
Keywords: CD47; RNA sequencing; capping protein inhibiting regulator of actin dynamics (CRACD); glioma; intraflagellar transport-57 (IFT57); lung adenocarcinoma; microsynteny; papillary thyroid carcinoma; primary cilium.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Mawby W.J., Holmes C.H., Anstee D.J., Spring F.A., Tanner M.J. Isolation and characterization of CD47 glycoprotein: A multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem. J. 1994;304:525–530. doi: 10.1042/bj3040525. - DOI - PMC - PubMed
-
- Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. - DOI - PMC - PubMed
-
- Baccelli I., Stenzinger A., Vogel V., Pfitzner B.M., Klein C., Wallwiener M., Scharpff M., Saini M., Holland-Letz T., Sinn H.P., et al. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget. 2014;5:8147–8160. doi: 10.18632/oncotarget.2385. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

