Functional and Structural Changes in Diaphragm Neuromuscular Junctions in Early Aging
- PMID: 39201644
- PMCID: PMC11354816
- DOI: 10.3390/ijms25168959
Functional and Structural Changes in Diaphragm Neuromuscular Junctions in Early Aging
Abstract
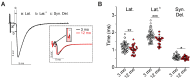
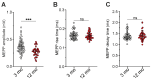
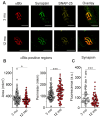
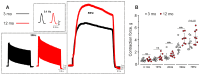
Age-related impairment of the diaphragm causes respiratory complications. Neuromuscular junction (NMJ) dysfunction can be one of the triggering events in diaphragm weaknesses in old age. Prominent structural and functional alterations in diaphragm NMJs were described in elderly rodents, but NMJ changes in middle age remain unclear. Here, we compared diaphragm muscles from young adult (3 months) and middle-aged (12 months) BALB/c mice. Microelectrode recordings, immunofluorescent staining, electron microscopy, myography, and whole-body plethysmography were used. We revealed presynaptic (i) and postsynaptic (ii) changes. The former (i) included an increase in both action potential propagation velocity and neurotransmitter release evoked by low-, moderate-, and high-frequency activity but a decrease in immunoexpression of synapsin 1 and synaptic vesicle clustering. The latter (ii) consisted of a decrease in currents via nicotinic acetylcholine receptors and the area of their distribution. These NMJ changes correlated with increased contractile responses to moderate- to high-frequency nerve activation. Additionally, we found alterations in the pattern of respiration (an increase in peak inspiratory flow and a tendency of elevation of the tidal volume), which imply increased diaphragm activity in middle-aged mice. We conclude that enhancement of neuromuscular communication (due to presynaptic mechanism) accompanied by improved contractile responses occurs in the diaphragm in early aging.
Keywords: acetylcholine; aging; contraction; diaphragm; end-plate; neuromuscular junction; neurotransmitter release; respiration; synapsin; synaptic vesicle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Siniscalchi C., Nouvenne A., Cerundolo N., Meschi T., Ticinesi A. On Behalf of The Parma Post-Graduate Specialization School in Emergency-Urgency Medicine Interest Group on Thoracic, U. Diaphragm Ultrasound in Different Clinical Scenarios: A Review with a Focus on Older Patients. Geriatrics. 2024;9:70. doi: 10.3390/geriatrics9030070. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

