Generic Reporter Sets for Colorimetric Multiplex dPCR Demonstrated with 6-Plex SNP Quantification Panels
- PMID: 39201654
- PMCID: PMC11355019
- DOI: 10.3390/ijms25168968
Generic Reporter Sets for Colorimetric Multiplex dPCR Demonstrated with 6-Plex SNP Quantification Panels
Abstract
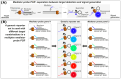
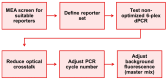
Digital PCR (dPCR) is a powerful method for highly sensitive and precise quantification of nucleic acids. However, designing and optimizing new multiplex dPCR assays using target sequence specific probes remains cumbersome, since fluorescent signals must be optimized for every new target panel. As a solution, we established a generic fluorogenic 6-plex reporter set, based on mediator probe technology, that decouples target detection from signal generation. This generic reporter set is compatible with different target panels and thus provides already optimized fluorescence signals from the start of new assay development. Generic reporters showed high population separability in a colorimetric 6-plex mediator probe dPCR, due to their tailored fluorophore and quencher selection. These reporters were further tested using different KRAS, NRAS and BRAF single-nucleotide polymorphisms (SNP), which are frequent point mutation targets in liquid biopsy. We specifically quantified SNP targets in our multiplex approach down to 0.4 copies per microliter (cp/µL) reaction mix, equaling 10 copies per reaction, on a wild-type background of 400 cp/µL for each, equaling 0.1% variant allele frequencies. We also demonstrated the design of an alternative generic reporter set from scratch in order to give detailed step-by-step guidance on how to systematically establish and optimize novel generic reporter sets. Those generic reporter sets can be customized for various digital PCR platforms or target panels with different degrees of multiplexing.
Keywords: BRAF; KRAS; NRAS; assay development; color compensation; digital PCR (dPCR); fluorophore combination; generic reporter set; mediator extension assay (MEA); mediator probe PCR (MP PCR); oncogenic mutations.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Reporter emission multiplexing in digital PCRs (REM-dPCRs): direct quantification of multiple target sequences per detection channel by population specific reporters.Analyst. 2023 Oct 5;148(20):5243-5254. doi: 10.1039/d3an00191a. Analyst. 2023. PMID: 37727114
-
Phase II study of high-sensitivity genotyping of KRAS, NRAS, BRAF and PIK3CA to ultra-select metastatic colorectal cancer patients for panitumumab plus FOLFIRI: the ULTRA trial.Ann Oncol. 2019 May 1;30(5):796-803. doi: 10.1093/annonc/mdz082. Ann Oncol. 2019. PMID: 30840064 Clinical Trial.
-
Development of Multiplex Drop-Off Digital PCR Assays for Hotspot Mutation Detection of KRAS, NRAS, BRAF, and PIK3CA in the Plasma of Colorectal Cancer Patients.J Mol Diagn. 2023 Jun;25(6):388-402. doi: 10.1016/j.jmoldx.2023.03.002. Epub 2023 Mar 22. J Mol Diagn. 2023. PMID: 36963484
-
Molecular diagnosis in type I epithelial ovarian cancer.Ginekol Pol. 2017;88(12):692-697. doi: 10.5603/GP.a2017.0123. Ginekol Pol. 2017. PMID: 29303228 Review.
-
The Coexistence of RAS and BRAF Mutations in Metastatic Colorectal Cancer: A Case Report and Systematic Literature Review.J Gastrointestin Liver Dis. 2020 Jun 3;29(2):251-256. doi: 10.15403/jgld-1003. J Gastrointestin Liver Dis. 2020. PMID: 32530992
Cited by
-
Digital PCR: from early developments to its future application in clinics.Lab Chip. 2025 Aug 5;25(16):3921-3961. doi: 10.1039/d5lc00055f. Lab Chip. 2025. PMID: 40686367 Free PMC article. Review.
-
Coupling Immunoprecipitation with Multiplexed Digital PCR for Cell-Free DNA Methylation Detection in Small Plasma Volumes of Early-Onset Colorectal Cancer.Anal Chem. 2025 Jun 3;97(21):11259-11268. doi: 10.1021/acs.analchem.5c01361. Epub 2025 May 17. Anal Chem. 2025. PMID: 40380352 Free PMC article.
References
-
- Hou Y., Chen S., Zheng Y., Zheng X., Lin J.-M. Droplet-based digital PCR (ddPCR) and its applications. TrAC Trends Anal. Chem. 2023;158:116897. doi: 10.1016/j.trac.2022.116897. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

