Expression and Functional Identification of SPL6/7/9 Genes under Drought Stress in Sugarbeet Seedlings
- PMID: 39201675
- PMCID: PMC11354545
- DOI: 10.3390/ijms25168989
Expression and Functional Identification of SPL6/7/9 Genes under Drought Stress in Sugarbeet Seedlings
Abstract
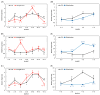
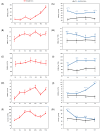
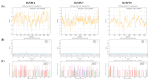
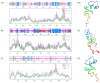
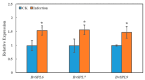
Sugar beet is a significant sugar crop in China, primarily cultivated in arid regions of the north. However, drought often affects sugar beet cultivation, leading to reduced yield and quality. Therefore, understanding the impact of drought on sugar beets and studying their drought tolerance is crucial. Previous research has examined the role of SPL (SQUAMOSA promoter-binding protein-like) transcription factors in plant stress response; however, the precise contribution of SPLs to the drought stress response in sugar beets has yet to be elucidated. In this study, we identified and examined the BvSPL6, BvSPL7, and BvSPL9 genes in sugar beets, investigating their performance during the seedling stage under drought stress. We explored their drought resistance characteristics using bioinformatics, quantitative analysis, physiological experiments, and molecular biology experiments. Drought stress and rehydration treatments were applied to sugar beet seedlings, and the expression levels of BvSPL6, BvSPL7, and BvSPL9 genes in leaves were quantitatively analyzed at 11 different time points to evaluate sugar beets' response and tolerance to drought stress. Results indicated that the expression level of the BvSPL6/9 genes in leaves was upregulated during the mid-stage of drought stress and downregulated during the early and late stages. Additionally, the expression level of the BvSPL7 gene gradually increased with the duration of drought stress. Through analyzing changes in physiological indicators during different time periods of drought stress and rehydration treatment, we speculated that the regulation of BvSPL6/7/9 genes is associated with sugar beet drought resistance and their participation in drought stress response. Furthermore, we cloned the CDS sequences of BvSPL6, BvSPL7, and BvSPL9 genes from sugar beets and conducted sequence alignment with the database to validate the results. Subsequently, we constructed overexpression vectors, named 35S::BvSPL6, 35S::BvSPL7, and 35S::BvSPL9, and introduced them into sugar beets using Agrobacterium-mediated methods. Real-time fluorescence quantitative analysis revealed that the expression levels of BvSPL6/7/9 genes in transgenic sugar beets increased by 40% to 80%. The drought resistance of transgenic sugar beets was significantly enhanced compared with the control group.
Keywords: Beta vulgaris L.; SPL; drought stress; plant stress resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Zhang Q., Yao Y., Li Y., Huang J., Ma Z., Wang Z., Wang S., Wang Y., Zhang Y. Causes and Changes of Drought in China: Research Progress and Prospects. J. Meteorol. Res. 2020;34:460–481. doi: 10.1007/s13351-020-9829-8. - DOI
-
- Yu X., Zhang L., Zhou T., Zhang X. Long-term changes in the effect of drought stress on ecosystems across global drylands. Sci. China Earth Sci. 2023;66:146–160. doi: 10.1007/s11430-022-1001-0. - DOI
-
- He F., Long R., Wei C., Zhang Y., Li M., Kang J., Yang Q., Wang Z., Chen L. Genome-wide identification, phylogeny and expression analysis of the SPL gene family and its important role in salt stress in Medicago sativa L. BMC Plant Biol. 2022;22:295. doi: 10.1186/s12870-022-03678-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

