A Simple Expression for the Screening of Excitonic Couplings between Chlorophylls as Inferred for Photosystem I Trimers
- PMID: 39201694
- PMCID: PMC11355009
- DOI: 10.3390/ijms25169006
A Simple Expression for the Screening of Excitonic Couplings between Chlorophylls as Inferred for Photosystem I Trimers
Abstract
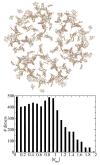
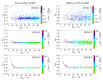
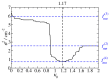
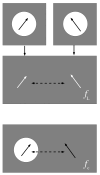
The Coulomb coupling between transition densities of the pigments in photosynthetic pigment-protein complexes, termed excitonic coupling, is a key factor for the description of optical spectra and energy transfer. A challenging question is the quantification of the screening of the excitonic coupling by the optical polarizability of the environment. We use the equivalence between the sophisticated quantum chemical polarizable continuum (PCM) model and the simple electrostatic Poisson-TrEsp approach to analyze the distance and orientation dependence of the dielectric screening between chlorophylls in photosystem I trimers. On the basis of these calculations we find that the vacuum couplings Vmn(0) and the couplings in the dielectric medium Vmn=fmnVmn(0) are related by the empirical screening factor fmn=0.60+39.6θ(|κmn|-1.17)exp(-0.56Rmn/Å), where κmn is the usual orientational factor of the dipole-dipole coupling between the pigments, Rmn is the center-to-center distance, and the Heaviside-function θ(|κmn|-1.17) ensures that the exponential distance dependence only contributes for in-line type dipole geometries. We are confident that the present expression can be applied also to other pigment-protein complexes with chlorophyll or related pigments of similar shape. The variance between the Poisson-TrEsp and the approximate coupling values is found to decrease by a factor of 8 and 3-4 using the present expression, instead of an exponential distance dependent or constant screening factor, respectively, assumed previously in the literature.
Keywords: chlorophylls; dielectric screening; excitonic couplings; light-harvesting antenna.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Xie H., Lyratzakis A., Khera R., Kountantou M., Welsch S., Michel H., Tsiotis G. Cryo-EM structure of the whole photosynthetic reaction center apparatus from the green sulfur bacterium Chlorobaculum tepidum. Proc. Natl. Acad. Sci. USA. 2023;120:e2216734120. doi: 10.1073/pnas.2216734120. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

