Antioxidant Role of Probiotics in Inflammation-Induced Colorectal Cancer
- PMID: 39201713
- PMCID: PMC11354872
- DOI: 10.3390/ijms25169026
Antioxidant Role of Probiotics in Inflammation-Induced Colorectal Cancer
Abstract
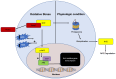
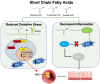
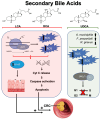
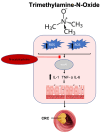
Colorectal cancer (CRC) continues to be a significant contributor to global morbidity and mortality. Emerging evidence indicates that disturbances in gut microbial composition, the formation of reactive oxygen species (ROS), and the resulting inflammation can lead to DNA damage, driving the pathogenesis and progression of CRC. Notably, bacterial metabolites can either protect against or contribute to oxidative stress by modulating the activity of antioxidant enzymes and influencing signaling pathways that govern ROS-induced inflammation. Additionally, microbiota byproducts, when supplemented through probiotics, can affect tumor microenvironments to enhance treatment efficacy and selectively mediate the ROS-induced destruction of CRC cells. This review aims to discuss the mechanisms by which taxonomical shifts in gut microbiota and related metabolites such as short-chain fatty acids, secondary bile acids, and trimethylamine-N-oxide influence ROS concentrations to safeguard or promote the onset of inflammation-mediated CRC. Additionally, we focus on the role of probiotic species in modulating ROS-mediated signaling pathways that influence both oxidative status and inflammation, such as Nrf2-Keap1, NF-κB, and NLRP3 to mitigate carcinogenesis. Overall, a deeper understanding of the role of gut microbiota on oxidative stress may aid in delaying or preventing the onset of CRC and offer new avenues for adjunct, CRC-specific therapeutic interventions such as cancer immunotherapy.
Keywords: antioxidants; bile acids; gut microbiota; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection.Int J Mol Sci. 2025 Apr 15;26(8):3733. doi: 10.3390/ijms26083733. Int J Mol Sci. 2025. PMID: 40332367 Free PMC article. Review.
-
Relationship between gut microbiota and colorectal cancer: Probiotics as a potential strategy for prevention.Food Res Int. 2022 Jun;156:111327. doi: 10.1016/j.foodres.2022.111327. Epub 2022 May 2. Food Res Int. 2022. PMID: 35651078 Review.
-
Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials.Int J Mol Sci. 2025 Mar 28;26(7):3148. doi: 10.3390/ijms26073148. Int J Mol Sci. 2025. PMID: 40243936 Free PMC article. Review.
-
The roles of microbial products in the development of colorectal cancer: a review.Bioengineered. 2021 Dec;12(1):720-735. doi: 10.1080/21655979.2021.1889109. Bioengineered. 2021. PMID: 33618627 Free PMC article. Review.
-
The role of gut microbiota and probiotics in preventing, treating, and boosting the immune system in colorectal cancer.Life Sci. 2024 May 1;344:122529. doi: 10.1016/j.lfs.2024.122529. Epub 2024 Mar 13. Life Sci. 2024. PMID: 38490297 Review.
Cited by
-
Targeting inflammation in cancer therapy: from mechanistic insights to emerging therapeutic approaches.J Transl Med. 2025 May 26;23(1):588. doi: 10.1186/s12967-025-06583-3. J Transl Med. 2025. PMID: 40420174 Free PMC article. Review.
-
Pathological and Inflammatory Consequences of Aging.Biomolecules. 2025 Mar 12;15(3):404. doi: 10.3390/biom15030404. Biomolecules. 2025. PMID: 40149940 Free PMC article. Review.
-
Saccharomyces cervisiae ameliorative impact combined with sulfaclozine on broiler chicken oxidative status.BMC Vet Res. 2025 Aug 6;21(1):507. doi: 10.1186/s12917-025-04955-x. BMC Vet Res. 2025. PMID: 40770331 Free PMC article.
References
-
- Patel S.G., Karlitz J.J., Yen T., Lieu C.H., Boland C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022;7:262–274. doi: 10.1016/S2468-1253(21)00426-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

