Synthesis of Natural and Sugar-Modified Nucleosides Using the Iodine/Triethylsilane System as N-Glycosidation Promoter
- PMID: 39201716
- PMCID: PMC11354600
- DOI: 10.3390/ijms25169030
Synthesis of Natural and Sugar-Modified Nucleosides Using the Iodine/Triethylsilane System as N-Glycosidation Promoter
Abstract
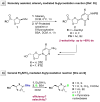
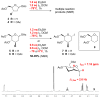
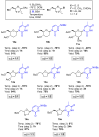
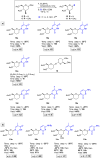
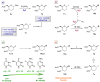
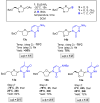
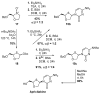
The reagent system based on the combined use of Et3SiH/I2 acts as an efficient N-glycosidation promoter for the synthesis of natural and sugar-modified nucleosides. An analysis of reaction stereoselectivity in the absence of C2-positioned stereodirecting groups revealed high selectivity with six-membered substrates, depending on the nucleophilic character of the nucleobase or based on anomerization reactions. The synthetic utility of the Et3SiH/I2-mediated N-glycosidation reaction was highlighted by its use in the synthesis of the investigational drug apricitabine.
Keywords: N-glycosidation; anomerization; apricitabine; glycosyl iodide; iodine/triethylsilane; nucleosides; stereoselectivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
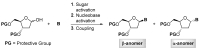
Similar articles
-
Tunable stereoselectivity in the synthesis of α- and β-aryl glycosides using 1,2-α-anhydrosugars as glycosyl donors.Carbohydr Res. 2017 Sep 8;449:95-102. doi: 10.1016/j.carres.2017.07.007. Epub 2017 Jul 25. Carbohydr Res. 2017. PMID: 28759815
-
Taming the Reactivity of Glycosyl Iodides To Achieve Stereoselective Glycosidation.Acc Chem Res. 2016 Jan 19;49(1):35-47. doi: 10.1021/acs.accounts.5b00357. Epub 2015 Nov 2. Acc Chem Res. 2016. PMID: 26524481
-
NIS/TMSOTf-Promoted Glycosidation of Glycosyl ortho-Hexynylbenzoates for Versatile Synthesis of O-Glycosides and Nucleosides.J Org Chem. 2021 Mar 19;86(6):4763-4778. doi: 10.1021/acs.joc.1c00151. Epub 2021 Mar 9. J Org Chem. 2021. PMID: 33689328
-
Glycosylation reactions mediated by hypervalent iodine: application to the synthesis of nucleosides and carbohydrates.Beilstein J Org Chem. 2018 Jun 28;14:1595-1618. doi: 10.3762/bjoc.14.137. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30013687 Free PMC article. Review.
-
Assembly of naturally occurring glycosides, evolved tactics, and glycosylation methods.Acc Chem Res. 2012 Aug 21;45(8):1227-36. doi: 10.1021/ar200296m. Epub 2012 Apr 11. Acc Chem Res. 2012. PMID: 22493991 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

