Wilson's Disease-Crossroads of Genetics, Inflammation and Immunity/Autoimmunity: Clinical and Molecular Issues
- PMID: 39201720
- PMCID: PMC11354778
- DOI: 10.3390/ijms25169034
Wilson's Disease-Crossroads of Genetics, Inflammation and Immunity/Autoimmunity: Clinical and Molecular Issues
Abstract
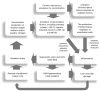
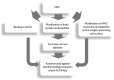
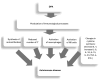
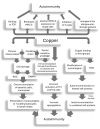
Wilson's disease (WD) is a rare, autosomal recessive disorder of copper metabolism caused by pathogenic mutations in the ATP7B gene. Cellular copper overload is associated with impaired iron metabolism. Oxidative stress, cuproptosis, and ferroptosis are involved in cell death in WD. The clinical picture of WD is variable. Hepatic/neuropsychiatric/other symptoms may manifest in childhood/adulthood and even old age. It has been shown that phenotypic variability may be determined by the type of ATP7B genetic variants as well as the influence of various genetic/epigenetic, environmental, and lifestyle modifiers. In 1976, immunological abnormalities were first described in patients with WD. These included an increase in IgG and IgM levels and a decrease in the percentage of T lymphocytes, as well as a weakening of their bactericidal effect. Over the following years, it was shown that there is a bidirectional relationship between copper and inflammation. Changes in serum cytokine concentrations and the relationship between cytokine gene variants and the clinical course of the disease have been described in WD patients, as well as in animal models of this disease. Data have also been published on the occurrence of antinuclear antibodies (ANAs), antineutrophil cytoplasmic antibodies (ANCAs), anti-muscle-specific tyrosine kinase antibodies, and anti-acetylcholine receptor antibodies, as well as various autoimmune diseases, including systemic lupus erythematosus (SLE), myasthenic syndrome, ulcerative colitis, multiple sclerosis (MS), polyarthritis, and psoriasis after treatment with d-penicillamine (DPA). The occurrence of autoantibodies was also described, the presence of which was not related to the type of treatment or the form of the disease (hepatic vs. neuropsychiatric). The mechanisms responsible for the occurrence of autoantibodies in patients with WD are not known. It has also not been clarified whether they have clinical significance. In some patients, WD was differentiated or coexisted with an autoimmune disease, including autoimmune hepatitis or multiple sclerosis. Various molecular mechanisms may be responsible for immunological abnormalities and/or the inflammatory processes in WD. Their better understanding may be important for explaining the reasons for the diversity of symptoms and the varied course and response to therapy, as well as for the development of new treatment regimens for WD.
Keywords: ATP7B; Wilson’s disease; autoantibodies; autoimmunity; copper; cuproptosis; ferroptosis; immunology; inflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

