The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature
- PMID: 39201722
- PMCID: PMC11354688
- DOI: 10.3390/ijms25169037
The Pathophysiological Mechanism and Clinical Treatment of Polycystic Ovary Syndrome: A Molecular and Cellular Review of the Literature
Abstract
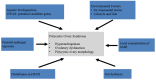
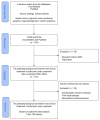
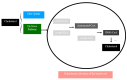
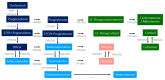
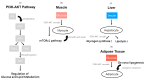
Polycystic ovary syndrome (PCOS) is a prevalent metabolic disorder among women of reproductive age, characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries. The pathogenesis of PCOS involves a complex interplay of genetic and environmental factors, including insulin resistance (IR) and resultant hyperinsulinemia. Insulin receptors, primarily in skeletal muscle, liver, and adipose tissue, activate downstream signaling pathways like PI3K-AKT and MAPK-ERK upon binding. These pathways regulate glucose uptake, storage, and lipid metabolism. Genome-wide association studies (GWASs) have identified several candidate genes related to steroidogenesis and insulin signaling. Environmental factors such as endocrine-disrupting chemicals and lifestyle choices also exacerbate PCOS traits. Other than lifestyle modification and surgical intervention, management strategies for PCOS can be achieved by using pharmacological treatments like antiandrogens, metformin, thiazolidinediones, aromatase inhibitor, and ovulation drugs to improve insulin sensitivity and ovulatory function, as well as combined oral contraceptives with or without cyproterone to resume menstrual regularity. Despite the complex pathophysiology and significant economic burden of PCOS, a comprehensive understanding of its molecular and cellular mechanisms is crucial for developing effective public health policies and treatment strategies. Nevertheless, many unknown aspects of PCOS, including detailed mechanisms of actions, along with the safety and effectiveness for the treatment, warrant further investigation.
Keywords: hyperandrogenism; hyperinsulinemia; insulin resistance; polycystic ovary syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Goh J.E., Farrukh M.J., Keshavarzi F., Yap C.S., Saleem Z., Salman A., John A. Assessment of Prevalence, Knowledge of Polycystic Ovary Syndrome and Health-related Practices among Women in Klang Valley: A Cross-sectional Survey. Front. Endocrinol. 2022;13:985588. doi: 10.3389/fendo.2022.985588. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

