Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile
- PMID: 39201723
- PMCID: PMC11354302
- DOI: 10.3390/ijms25169029
Effects of Fasting on THP1 Macrophage Metabolism and Inflammatory Profile
Abstract
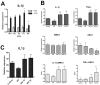
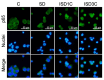
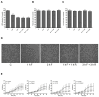
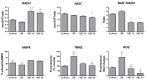
Fasting can affect the body's inflammatory response, and this has been linked to potential health benefits, including improvements for people with rheumatic diseases. In this work, we evaluated, in vitro, how changes in nutrient availability alter the inflammatory response of macrophages. Macrophage-differentiated THP1 cells were cultured, deprived of FCS or subjected to cycles of FCS deprivation and restoration to mimic intermittent fasting. Changes in the macrophage phenotype, the cells' response to inflammatory stimuli and the level of mitochondrial alteration were assessed. The results indicate that while periods of serum starvation are associated with a decrease in IL1β and TNFα expression, consistent with an anti-inflammatory response, intermittent serum starvation cycles promote a pro-inflammatory phenotype. Rapid changes in reducing capacity and mitochondrial response were also observed. Of note, while some changes, such as the production of oxygen free radicals, were reversed with refeeding, others, such as a decrease in reducing capacity, were maintained and even increased. This study shows that different fasting protocols can have diverging effects and highlights that time-limited nutrient changes can significantly affect macrophage functions in cell cultures. These findings help elucidate some of the mechanisms by which specific fasting dietary interventions could help control inflammatory diseases.
Keywords: fasting; inflammation; intermittent fasting; macrophages; rheumatic diseases.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Total saponin of Dioscorea collettii attenuates MSU crystal‑induced inflammation via inhibiting the activation of the NALP3 inflammasome and caspase‑1 in THP‑1 macrophages.Mol Med Rep. 2020 Jun;21(6):2466-2474. doi: 10.3892/mmr.2020.11035. Epub 2020 Mar 20. Mol Med Rep. 2020. PMID: 32236574 Free PMC article.
-
Ferulic acid but not alpha-lipoic acid effectively protects THP-1-derived macrophages from oxidant and pro-inflammatory response to LPS.Immunopharmacol Immunotoxicol. 2017 Dec;39(6):330-337. doi: 10.1080/08923973.2017.1369100. Epub 2017 Sep 5. Immunopharmacol Immunotoxicol. 2017. PMID: 28872362
-
Intermittent High Glucose Exacerbates A-FABP Activation and Inflammatory Response through TLR4-JNK Signaling in THP-1 Cells.J Immunol Res. 2018 Apr 11;2018:1319272. doi: 10.1155/2018/1319272. eCollection 2018. J Immunol Res. 2018. PMID: 29850615 Free PMC article.
-
Mitoimmunity-when mitochondria dictates macrophage function.Cell Biol Int. 2018 Jun;42(6):651-655. doi: 10.1002/cbin.10921. Epub 2018 Jan 22. Cell Biol Int. 2018. PMID: 29271525 Review.
-
Mitochondrial metabolism, reactive oxygen species, and macrophage function-fishing for insights.J Mol Med (Berl). 2014 Nov;92(11):1119-28. doi: 10.1007/s00109-014-1186-6. Epub 2014 Jun 25. J Mol Med (Berl). 2014. PMID: 24957262 Review.
References
-
- Dessein P.H., Shipton E.A., Stanwix A.E., Joffe B.I., Ramokgadi J. Beneficial Effects of Weight Loss Associated with Moderate Calorie/Carbohydrate Restriction, and Increased Proportional Intake of Protein and Unsaturated Fat on Serum Urate and Lipoprotein Levels in Gout: A Pilot Study. Ann. Rheum. Dis. 2000;59:539–543. doi: 10.1136/ard.59.7.539. - DOI - PMC - PubMed
-
- Gwinnutt J.M., Wieczorek M., Balanescu A., Bischoff-Ferrari H.A., Boonen A., Cavalli G., de Souza S., de Thurah A., Dorner T.E., Moe R.H., et al. 2021 EULAR Recommendations Regarding Lifestyle Behaviours and Work Participation to Prevent Progression of Rheumatic and Musculoskeletal Diseases. Ann. Rheum. Dis. 2023;82:48–56. doi: 10.1136/annrheumdis-2021-222020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

