Quercetin Attenuates Acetaldehyde-Induced Cytotoxicity via the Heme Oxygenase-1-Dependent Antioxidant Mechanism in Hepatocytes
- PMID: 39201725
- PMCID: PMC11354654
- DOI: 10.3390/ijms25169038
Quercetin Attenuates Acetaldehyde-Induced Cytotoxicity via the Heme Oxygenase-1-Dependent Antioxidant Mechanism in Hepatocytes
Abstract
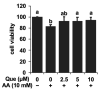
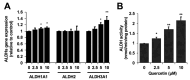
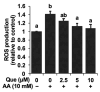
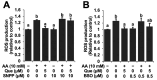
It is still unclear whether or how quercetin influences the toxic events induced by acetaldehyde in hepatocytes, though quercetin has been reported to mitigate alcohol-induced mouse liver injury. In this study, we evaluated the modulating effect of quercetin on the cytotoxicity induced by acetaldehyde in mouse hepatoma Hepa1c1c7 cells, the frequently used cellular hepatocyte model. The pretreatment with quercetin significantly inhibited the cytotoxicity induced by acetaldehyde. The treatment with quercetin itself had an ability to enhance the total ALDH activity, as well as the ALDH1A1 and ALDH3A1 gene expressions. The acetaldehyde treatment significantly enhanced the intracellular reactive oxygen species (ROS) level, whereas the quercetin pretreatment dose-dependently inhibited it. Accordingly, the treatment with quercetin itself significantly up-regulated the representative intracellular antioxidant-related gene expressions, including heme oxygenase-1 (HO-1), glutamate-cysteine ligase, catalytic subunit (GCLC), and cystine/glutamate exchanger (xCT), that coincided with the enhancement of the total intracellular glutathione (GSH) level. Tin protoporphyrin IX (SNPP), a typical HO-1 inhibitor, restored the quercetin-induced reduction in the intracellular ROS level, whereas buthionine sulphoximine, a representative GSH biosynthesis inhibitor, did not. SNPP also cancelled the quercetin-induced cytoprotection against acetaldehyde. These results suggest that the low-molecular-weight antioxidants produced by the HO-1 enzymatic reaction are mainly attributable to quercetin-induced cytoprotection.
Keywords: acetaldehyde; aldehyde dehydrogenase; glutathione; heme oxygenase-1; quercetin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The microbiota catabolites of quercetin glycosides concertedly enhance the resistance against acetaldehyde-induced oxidative stress.Free Radic Res. 2022 Sep-Oct;56(9-10):607-616. doi: 10.1080/10715762.2022.2159820. Epub 2023 Jan 3. Free Radic Res. 2022. PMID: 36576903
-
Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes.Arch Toxicol. 2013 Jan;87(1):167-78. doi: 10.1007/s00204-012-0913-4. Epub 2012 Aug 5. Arch Toxicol. 2013. PMID: 22864849
-
Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes.Toxicol Appl Pharmacol. 2014 Jan 15;274(2):293-301. doi: 10.1016/j.taap.2013.10.026. Epub 2013 Nov 7. Toxicol Appl Pharmacol. 2014. PMID: 24211276
-
A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity.Int J Mol Sci. 2022 Feb 3;23(3):1762. doi: 10.3390/ijms23031762. Int J Mol Sci. 2022. PMID: 35163684 Free PMC article.
-
Evaluation of quercetin as a potential cytoprotector against acetaldehyde using the cultured hepatocyte model with aldehyde dehydrogenase isozyme deficiency.Biosci Biotechnol Biochem. 2024 Sep 20;88(10):1199-1202. doi: 10.1093/bbb/zbae100. Biosci Biotechnol Biochem. 2024. PMID: 38991992
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

