The Synergistic Effects of Polyol Pathway-Induced Oxidative and Osmotic Stress in the Aetiology of Diabetic Cataracts
- PMID: 39201727
- PMCID: PMC11354722
- DOI: 10.3390/ijms25169042
The Synergistic Effects of Polyol Pathway-Induced Oxidative and Osmotic Stress in the Aetiology of Diabetic Cataracts
Abstract
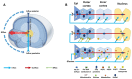
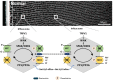
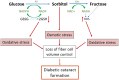
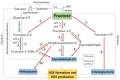
Cataracts are the world's leading cause of blindness, and diabetes is the second leading risk factor for cataracts after old age. Despite this, no preventative treatment exists for cataracts. The altered metabolism of excess glucose during hyperglycaemia is known to be the underlying cause of diabetic cataractogenesis, resulting in localised disruptions to fibre cell morphology and cell swelling in the outer cortex of the lens. In rat models of diabetic cataracts, this damage has been shown to result from osmotic stress and oxidative stress due to the accumulation of intracellular sorbitol, the depletion of NADPH which is used to regenerate glutathione, and the generation of fructose metabolites via the polyol pathway. However, differences in lens physiology and the metabolism of glucose in the lenses of different species have prevented the translation of successful treatments in animal models into effective treatments in humans. Here, we review the stresses that arise from hyperglycaemic glucose metabolism and link these to the regionally distinct metabolic and physiological adaptations in the lens that are vulnerable to these stressors, highlighting the evidence that chronic oxidative stress together with osmotic stress underlies the aetiology of human diabetic cortical cataracts. With this information, we also highlight fundamental gaps in the knowledge that could help to inform new avenues of research if effective anti-diabetic cataract therapies are to be developed in the future.
Keywords: diabetic cataract; glucose metabolism; osmotic stress; oxidative stress; polyol pathway; volume regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lehtovirta M., Pietiläinen K.H., Levälahti E., Heikkilä K., Groop L., Silventoinen K., Koskenvuo M., Kaprio J. Evidence that BMI and type 2 diabetes share only a minor fraction of genetic variance: A follow-up study of 23,585 monozygotic and dizygotic twins from the Finnish Twin Cohort Study. Diabetologia. 2010;53:1314–1321. doi: 10.1007/s00125-010-1746-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

