Advances in Cholinesterase Inhibitor Research-An Overview of Preclinical Studies of Selected Organoruthenium(II) Complexes
- PMID: 39201735
- PMCID: PMC11354293
- DOI: 10.3390/ijms25169049
Advances in Cholinesterase Inhibitor Research-An Overview of Preclinical Studies of Selected Organoruthenium(II) Complexes
Abstract
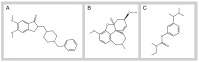
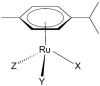
Cholinesterase (ChE) inhibitors are crucial therapeutic agents for the symptomatic treatment of certain chronic neurodegenerative diseases linked to functional disorders of the cholinergic system. Significant research efforts have been made to develop novel derivatives of classical ChE inhibitors and ChE inhibitors with novel scaffolds. Over the past decade, ruthenium complexes have emerged as promising novel therapeutic alternatives for the treatment of neurodegenerative diseases. Our research group has investigated a number of newly synthesized organoruthenium(II) complexes for their inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Three complexes (C1a, C1-C, and C1) inhibit ChE in a pharmacologically relevant range. C1a reversibly inhibits AChE and BChE without undesirable peripheral effects, making it a promising candidate for the treatment of Alzheimer's disease. C1-Cl complex reversibly and competitively inhibits ChEs, particularly AChE. It inhibits nerve-evoked skeletal muscle twitch and tetanic contraction in a concentration-dependent manner with no effect on directly elicited twitch and tetanic contraction and is promising for further preclinical studies as a competitive neuromuscular blocking agent. C1 is a selective, competitive, and reversible inhibitor of BChE that inhibits horse serum BChE (hsBChE) without significant effect on the peripheral neuromuscular system and is a highly species-specific inhibitor of hsBChE that could serve as a species-specific drug target. This research contributes to the expanding knowledge of ChE inhibitors based on ruthenium complexes and highlights their potential as promising therapeutic candidates for chronic neurodegenerative diseases.
Keywords: cholinesterases; enzyme inhibition; mouse; neuromuscular junction; organoruthenium complex.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
References
-
- Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141:1917–1933. doi: 10.1093/brain/awy132. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

