Post-Acute Sequelae and Mitochondrial Aberration in SARS-CoV-2 Infection
- PMID: 39201736
- PMCID: PMC11354507
- DOI: 10.3390/ijms25169050
Post-Acute Sequelae and Mitochondrial Aberration in SARS-CoV-2 Infection
Abstract
This review investigates links between post-acute sequelae of SARS-CoV-2 infection (PASC), post-infection viral persistence, mitochondrial involvement and aberrant innate immune response and cellular metabolism during SARS-CoV-2 infection. Advancement of proteomic and metabolomic studies now allows deeper investigation of alterations to cellular metabolism, autophagic processes and mitochondrial dysfunction caused by SARS-CoV-2 infection, while computational biology and machine learning have advanced methodologies of predicting virus-host gene and protein interactions. Particular focus is given to the interaction between viral genes and proteins with mitochondrial function and that of the innate immune system. Finally, the authors hypothesise that viral persistence may be a function of mitochondrial involvement in the sequestration of viral genetic material. While further work is necessary to understand the mechanisms definitively, a number of studies now point to the resolution of questions regarding the pathogenesis of PASC.
Keywords: PASC; SARS-CoV-2; autophagy; cell metabolism; innate immunity; long COVID; mitochondria; mitophagy; mtDNA; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
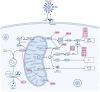
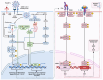
References
-
- Post COVID-19 Condition (Long COVID) [(accessed on 30 January 2024)]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-cond....
-
- Overview|COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19|Guidance|NICE. [(accessed on 30 January 2024)]. Available online: https://www.nice.org.uk/guidance/NG188.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

