Intracellular Survival and Pathogenicity Modulation of Salmonella Lon, CpxR, and RfaL Mutants Used as Live Bacterial Vectors under Abiotic Stress, Unveiling the Link between Stress Response and Virulence in Epithelial Cells
- PMID: 39201742
- PMCID: PMC11354574
- DOI: 10.3390/ijms25169056
Intracellular Survival and Pathogenicity Modulation of Salmonella Lon, CpxR, and RfaL Mutants Used as Live Bacterial Vectors under Abiotic Stress, Unveiling the Link between Stress Response and Virulence in Epithelial Cells
Abstract
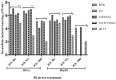
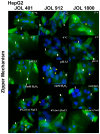
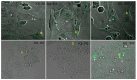
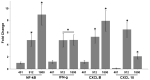
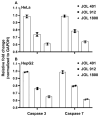
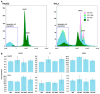
In the current study, two Salmonella Typhimurium strains, JOL 912 and JOL 1800, were engineered from the wild-type JOL 401 strain through in-frame deletions of the lon and cpxR genes, with JOL 1800 also lacking rfaL. These deletions significantly attenuated the strains, impairing their intracellular survival and creating unique immunological profiles. This study investigates the response of these strains to various abiotic stress conditions commonly experienced in vivo, including temperature, acidity, osmotic, and oxidative stress. Notably, cold stress induced a non-significant trend towards increased invasion by Salmonella compared to other stressors. Despite the observed attenuation, no significant alterations in entry mechanisms (trigger vs. zipper) were noted between these strains, although variations were evident depending on the host cell type. Both strains effectively localized within the cytoplasm, demonstrating their ability to invade and interact with the intracellular environment. Immunologically, JOL 912 elicited a robust response, marked by substantial activation of nuclear factor kappa B (NF-kB), and chemokines, interleukin 8 (CXCL 8) and interleukin 10 (CXCL 10), comparable to the wild-type JOL 401 (over a fourfold increase compared to JOL 1800). In contrast, JOL 1800 exhibited a minimal immune response. Additionally, these attenuations influenced the expression of cyclins D1 and B1 and caspases 3 and 7, indicating cell cycle arrest at the G2/M phase and promotion of the G0/G1 to S phase transition, alongside apoptosis in infected cells. These findings provide valuable insights into the mechanisms governing the association, internalization, and survival of Salmonella mutants, enhancing our understanding of their regulatory effects on host cell physiology.
Keywords: NF-kB; Salmonella Typhimurium; abiotic stress; cell cycle arrest; chemokines; intracellular survival.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sereme Y., Schrimp C., Faury H., Agapoff M., Lefebvre-Wloszczowski E., Chang Marchand Y., Ageron-Ardila E., Panafieu E., Blec F., Coureuil M., et al. A live attenuated vaccine to prevent severe neonatal Escherichia coli K1 infections. Nat. Commun. 2024;15:3021. doi: 10.1038/s41467-024-46775-x. - DOI - PMC - PubMed
-
- Hochnadel I., Hoenicke L., Petriv N., Neubert L., Reinhard E., Hirsch T., Alfonso J.C.L., Suo H., Longerich T., Geffers R., et al. Safety and efficacy of prophylactic and therapeutic vaccine based on live-attenuated Listeria monocytogenes in hepatobiliary cancers. Oncogene. 2022;41:2039–2053. doi: 10.1038/s41388-022-02222-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

