AAV-Mediated Expression of miR-17 Enhances Neurite and Axon Regeneration In Vitro
- PMID: 39201743
- PMCID: PMC11355044
- DOI: 10.3390/ijms25169057
AAV-Mediated Expression of miR-17 Enhances Neurite and Axon Regeneration In Vitro
Abstract
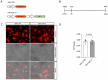
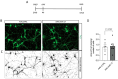
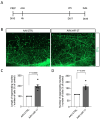
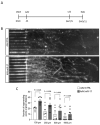
Neurodegenerative disorders, including traumatic injuries to the central nervous system (CNS) and neurodegenerative diseases, are characterized by early axonal damage, which does not regenerate in the adult mammalian CNS, leading to permanent neurological deficits. One of the primary causes of the loss of regenerative ability is thought to be a developmental decline in neurons' intrinsic capability for axon growth. Different molecules are involved in the developmental loss of the ability for axon regeneration, including many transcription factors. However, the function of microRNAs (miRNAs), which are also modulators of gene expression, in axon re-growth is still unclear. Among the various miRNAs recently identified with roles in the CNS, miR-17, which is highly expressed during early development, emerges as a promising target to promote axon regeneration. Here, we used adeno-associated viral (AAV) vectors to overexpress miR-17 (AAV.miR-17) in primary cortical neurons and evaluate its effects on neurite and axon regeneration in vitro. Although AAV.miR-17 had no significant effect on neurite outgrowth and arborization, it significantly enhances neurite regeneration after scratch lesion and axon regeneration after axotomy of neurons cultured in microfluidic chambers. Target prediction and functional annotation analyses suggest that miR-17 regulates gene expression associated with autophagy and cell metabolism. Our findings suggest that miR-17 promotes regenerative response and thus could mitigate neurodegenerative effects.
Keywords: axon; damage; miR-17; regeneration.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Bouslama-Oueghlani L., Wehrle R., Sotelo C., Dusart I. The Developmental Loss of the Ability of Purkinje Cells to Regenerate Their Axons Occurs in the Absence of Myelin: Na In Vitro Model to Prevent Myelination. J. Neurosci. 2003;23:8318–8329. doi: 10.1523/JNEUROSCI.23-23-08318.2003. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

