The Unexpected Role of the Endothelial Nitric Oxide Synthase at the Neurovascular Unit: Beyond the Regulation of Cerebral Blood Flow
- PMID: 39201757
- PMCID: PMC11354477
- DOI: 10.3390/ijms25169071
The Unexpected Role of the Endothelial Nitric Oxide Synthase at the Neurovascular Unit: Beyond the Regulation of Cerebral Blood Flow
Abstract
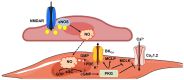
Nitric oxide (NO) is a highly versatile gasotransmitter that has first been shown to regulate cardiovascular function and then to exert tight control over a much broader range of processes, including neurotransmitter release, neuronal excitability, and synaptic plasticity. Endothelial NO synthase (eNOS) is usually far from the mind of synaptic neurophysiologists, who have focused most of their attention on neuronal NO synthase (nNOS) as the primary source of NO at the neurovascular unit (NVU). Nevertheless, the available evidence suggests that eNOS could also contribute to generating the burst of NO that, serving as volume intercellular messenger, is produced in response to neuronal activity in the brain parenchyma. Herein, we review the role of eNOS in both the regulation of cerebral blood flow and of synaptic plasticity and discuss the mechanisms by which cerebrovascular endothelial cells may transduce synaptic inputs into a NO signal. We further suggest that eNOS could play a critical role in vascular-to-neuronal communication by integrating signals converging onto cerebrovascular endothelial cells from both the streaming blood and active neurons.
Keywords: cerebrovascular endothelial cells; endothelial nitric oxide synthase; long-term potentiation; neurovascular coupling; neurovascular unit; nitric oxide; vascular-to-neuronal communication.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cycl. Nucleotide Res. 1977;3:23–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)-a multiscale in-tegrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022)/#NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR)
- "Support for research infrastructures considered critical/crucial for regional systems" Nuova Piattaforma di Farmacologia Integrata e Tecnologie Avanzate/European Commission's FESR FSE 2014-2020 and POR CALABRIA FESR AZIONE 1.5.1
- CVU121216/National Council of Science and Technology (CONACYT)
LinkOut - more resources
Full Text Sources

