Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia
- PMID: 39201760
- PMCID: PMC11354638
- DOI: 10.3390/ijms25169073
Oxysterol Induces Expression of 60 kDa Chaperone Protein on Cell Surface of Microglia
Abstract
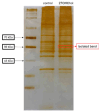
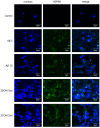
Microglia, essential immune cells in the brain, play crucial roles in neuroinflammation by performing various functions such as neurogenesis, synaptic pruning, and pathogen defense. These cells are activated by inflammatory factors like β-amyloid (Aβ) and oxysterols, leading to morphological and functional changes, including the secretion of inflammatory cytokines and the upregulation of MHC class II molecules. This study focused on identifying specific markers for microglial activation, with a particular emphasis on the roles of oxysterols in this process. We used the HMC3 human microglial cell line to investigate the induction of heat shock protein 60 (HSP60), a chaperonin protein by oxysterols, specifically in the presence of 25-hydroxycholesterol (25OHChol) and 27-hydroxycholesterol (27OHChol). Our findings obtained by the proteomics approach revealed that these oxysterols significantly increased HSP60 expression on microglial cells. This induction was further confirmed using Western blot analysis and immunofluorescence microscopy. Additionally, Aβ1-42 also promoted HSP60 expression, indicating its role as a microglial activator. HSP60 involved in protein folding and immune modulation was identified as a potential marker for microglial activation. This study underscores the importance of HSP60 in the inflammatory response of microglia, suggesting its utility as a target for new therapeutic approaches in neuroinflammatory diseases such as Alzheimer's disease (AD).
Keywords: 25-hydroxycholesterol; 27-hydroxycholesterol; HSP60; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

