Transglutaminase 2 Regulates HSF1 Gene Expression in the Acute Phase of Fish Optic Nerve Regeneration
- PMID: 39201764
- PMCID: PMC11354351
- DOI: 10.3390/ijms25169078
Transglutaminase 2 Regulates HSF1 Gene Expression in the Acute Phase of Fish Optic Nerve Regeneration
Abstract
Fish retinal ganglion cells (RGCs) can regenerate after optic nerve lesions (ONLs). We previously reported that heat shock factor 1 (HSF1) and Yamanaka factors increased in the zebrafish retina 0.5-24 h after ONLs, and they led to cell survival and the transformation of neuro-stem cells. We also showed that retinoic acid (RA) signaling and transglutaminase 2 (TG2) were activated in the fish retina, performing neurite outgrowth 5-30 days after ONLs. In this study, we found that RA signaling and TG2 increased within 0.5 h in the zebrafish retina after ONLs. We examined their interaction with the TG2-specific morpholino and inhibitor due to the significantly close initiation time of TG2 and HSF1. The inhibition of TG2 led to the complete suppression of HSF1 expression. Furthermore, the results of a ChIP assay with an anti-TG2 antibody evidenced significant anti-TG2 immunoprecipitation of HSF1 genome DNA after ONLs. The inhibition of TG2 also suppressed Yamanaka factors' gene expression. This rapid increase in TG2 expression occurred 30 min after the ONLs, and RA signaling occurred 15 min before this change. The present study demonstrates that TG2 regulates Yamanaka factors via HSF1 signals in the acute phase of fish optic nerve regeneration.
Keywords: HSF1; Klf4; Oct4; Sox2; TG2; Yamanaka factors; cell survival; optic nerve regeneration; retina; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

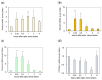
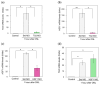




References
-
- Attardi D.G., Sperry R.W. Preferential selection of central pathways by regenerating optic fibers. Exp. Neurol. 1963;7:46–64. - PubMed
-
- Sperry R.W. Patterning of central synapses in regeneration of the optic nerve in teleosts. Physiol. Zool. 1948;21:351–361. - PubMed
-
- Bastmeyer M., Bähr M., Stuermer C.A. Fish Optic Nerve Oligodendrocytes Support Axonal Regeneration of Fish and Mammalian Retinal Ganglion Cells. Glia. 1993;8:1–11. - PubMed
-
- Becker T., Becker C.G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 2014;27:186–191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

