Aloperine Inhibits ASFV via Regulating PRLR/JAK2 Signaling Pathway In Vitro
- PMID: 39201769
- PMCID: PMC11354989
- DOI: 10.3390/ijms25169083
Aloperine Inhibits ASFV via Regulating PRLR/JAK2 Signaling Pathway In Vitro
Abstract
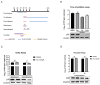
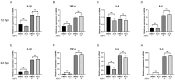
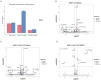
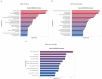
African swine fever (ASF) has become a global pandemic due to inadequate prevention and control measures, posing a significant threat to the swine industry. Despite the approval of a single vaccine in Vietnam, no antiviral drugs against the ASF virus (ASFV) are currently available. Aloperine (ALO), a quinolizidine alkaloid extracted from the seeds and leaves of bitter beans, exhibits various biological functions, including anti-inflammatory, anti-cancer, and antiviral activities. In this study, we found that ALO could inhibit ASFV replication in MA-104, PK-15, 3D4/21, and WSL cells in a dose-dependent manner without cytotoxicity at 100 μM. Furthermore, it was verified that ALO acted on the co- and post-infection stages of ASFV by time-of-addition assay, and inhibited viral internalization rather than directly inactivating the virus. Notably, RT-qPCR analysis indicated that ALO did not exert anti-inflammatory activity during ASFV infection. Additionally, gene ontology (GO) and KEGG pathway enrichment analyses of transcriptomic data revealed that ALO could inhibit ASFV replication via the PRLR/JAK2 signaling pathway. Together, these findings suggest that ALO effectively inhibits ASFV replication in vitro and provides a potential new target for developing anti-ASFV drugs.
Keywords: African swine fever virus; JAK2 signaling pathway; PRLR; aloperine; transcriptomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Zhang R., Huang Y., Bao C., Jung Y., Xu J., Qian Y. Epidemiology of African swine fever and analysis of risk factors of its spread in China: An overview. Chin. J. Virol. 2019;35:512–522.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

