Clinical Properties and Non-Clinical Testing of Mineralocorticoid Receptor Antagonists in In Vitro Cell Models
- PMID: 39201774
- PMCID: PMC11354261
- DOI: 10.3390/ijms25169088
Clinical Properties and Non-Clinical Testing of Mineralocorticoid Receptor Antagonists in In Vitro Cell Models
Abstract
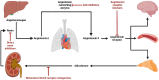
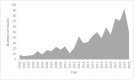
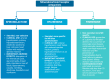
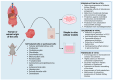
Mineralocorticoid receptor antagonists (MRAs) are one of the renin-angiotensin-aldosterone system inhibitors widely used in clinical practice. While spironolactone and eplerenone have a long-standing profile in clinical medicine, finerenone is a novel agent within the MRA class. It has a higher specificity for mineralocorticoid receptors, eliciting less pronounced adverse effects. Although approved for clinical use in patients with chronic kidney disease and heart failure, intensive non-clinical research aims to further elucidate its mechanism of action, including dose-related selectivity. Within the field, animal models remain the gold standard for non-clinical testing of drug pharmacological and toxicological properties. Their role, however, has been challenged by recent advances in in vitro models, mainly through sophisticated analytical tools and developments in data analysis. Currently, in vitro models are gaining momentum as possible platforms for advanced pharmacological and pathophysiological studies. This article focuses on past, current, and possibly future in vitro cell models research with clinically relevant MRAs.
Keywords: eplerenone; finerenone; in vitro; mineralocorticoid receptor antagonists; spironolactone.
Conflict of interest statement
S.B. has received a research grant and speaking honorarium from Bayer. The other authors have no conflicts of interest to declare.
Figures
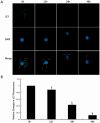
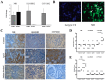
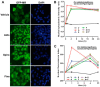
References
-
- Brown M.J. Direct renin inhibition—A new way of targeting the renin system. J. Renin-Angiotensin-Aldosterone Syst. 2006;7:S7–S11. doi: 10.3317/jraas.2006.035. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

