Inhibition of Calcineurin with FK506 Reduces Tau Levels and Attenuates Synaptic Impairment Driven by Tau Oligomers in the Hippocampus of Male Mouse Models
- PMID: 39201779
- PMCID: PMC11354963
- DOI: 10.3390/ijms25169092
Inhibition of Calcineurin with FK506 Reduces Tau Levels and Attenuates Synaptic Impairment Driven by Tau Oligomers in the Hippocampus of Male Mouse Models
Abstract
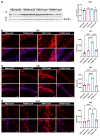
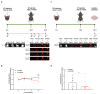
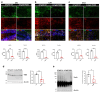
Alzheimer's disease (AD) is the most common age-associated neurodegenerative disorder, characterized by progressive cognitive decline, memory impairment, and structural brain changes, primarily involving Aβ plaques and neurofibrillary tangles of hyperphosphorylated tau protein. Recent research highlights the significance of smaller Aβ and Tau oligomeric aggregates (AβO and TauO, respectively) in synaptic dysfunction and disease progression. Calcineurin (CaN), a key calcium/calmodulin-dependent player in regulating synaptic function in the central nervous system (CNS) is implicated in mediating detrimental effects of AβO on synapses and memory function in AD. This study aims to investigate the specific impact of CaN on both exogenous and endogenous TauO through the acute and chronic inhibition of CaN. We previously demonstrated the protective effect against AD of the immunosuppressant CaN inhibitor, FK506, but its influence on TauO remains unclear. In this study, we explored the short-term effects of acute CaN inhibition on TauO phosphorylation and TauO-induced memory deficits and synaptic dysfunction. Mice received FK506 post-TauO intracerebroventricular injection and TauO levels and phosphorylation were assessed, examining their impact on CaN and GSK-3β. The study investigated FK506 preventive/reversal effects on TauO-induced clustering of CaN and GSK-3β. Memory and synaptic function in TauO-injected mice were evaluated with/without FK506. Chronic FK506 treatment in 3xTgAD mice explored its influence on CaN, Aβ, and Tau levels. This study underscores the significant influence of CaN inhibition on TauO and associated AD pathology, suggesting therapeutic potential in targeting CaN for addressing various aspects of AD onset and progression. These findings provide valuable insights for potential interventions in AD, emphasizing the need for further exploration of CaN-targeted strategies.
Keywords: Alzheimer’s disease; FK506; Tau; calcineurin; oligomers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- M Ashraf G., H Greig N., A Khan T., Hassan I., Tabrez S., Shakil S., A Sheikh I., K Zaidi S., Akram M., R Jabir N., et al. Protein Misfolding and Aggregation in Alzheimer’s Disease and Type 2 Diabetes Mellitus HHS Public Access. CNS Neurol. Disord. Drug. Targets. 2014;13:1280–1293. doi: 10.2174/1871527313666140917095514. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

