The Impact of the Major Endoribonucleases RNase E and RNase III and of the sRNA StsR on Photosynthesis Gene Expression in Rhodobacter sphaeroides Is Growth-Phase-Dependent
- PMID: 39201809
- PMCID: PMC11354728
- DOI: 10.3390/ijms25169123
The Impact of the Major Endoribonucleases RNase E and RNase III and of the sRNA StsR on Photosynthesis Gene Expression in Rhodobacter sphaeroides Is Growth-Phase-Dependent
Abstract
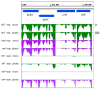
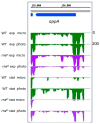
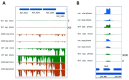
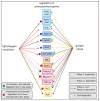
Rhodobacter sphaeroides is a facultative phototrophic bacterium that performs aerobic respiration when oxygen is available. Only when oxygen is present at low concentrations or absent are pigment-protein complexes formed, and anoxygenic photosynthesis generates ATP. The regulation of photosynthesis genes in response to oxygen and light has been investigated for decades, with a focus on the regulation of transcription. However, many studies have also revealed the importance of regulated mRNA processing. This study analyzes the phenotypes of wild type and mutant strains and compares global RNA-seq datasets to elucidate the impact of ribonucleases and the small non-coding RNA StsR on photosynthesis gene expression in Rhodobacter. Most importantly, the results demonstrate that, in particular, the role of ribonuclease E in photosynthesis gene expression is strongly dependent on growth phase.
Keywords: RNase E; RNase III; Rhodobacter; bacterial photosynthesis; pigment synthesis; ribonucleases; riboregulation; stationary phase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

