A Pilot Phase 2 Randomized Trial to Evaluate the Safety and Potential Efficacy of Etravirine in Friedreich Ataxia Patients
- PMID: 39201893
- PMCID: PMC11352957
- DOI: 10.3390/children11080958
A Pilot Phase 2 Randomized Trial to Evaluate the Safety and Potential Efficacy of Etravirine in Friedreich Ataxia Patients
Abstract
Background: A drug repositioning effort supported the possible use of the anti-HIV drug etravirine as a disease-modifying drug for Friedreich ataxia (FRDA). Etravirine increases frataxin protein and corrects the biochemical defects in cells derived from FRDA patients. Because of these findings, and since etravirine displays a favorable safety profile, we conducted a pilot open-label phase 2 clinical trial assessing the safety and potential efficacy of etravirine in FRDA patients.
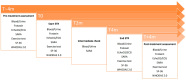
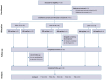
Methods: Thirty-five patients were stratified into three severity groups and randomized to etravirine 200 mg/day or 400 mg/day. They were treated for 4 months. Safety endpoints were the number and type of adverse events and number of dropouts. Efficacy endpoints were represented by changes in peak oxygen uptake and workload as measured by incremental exercise test, SARA score, cardiac measures, measures of QoL and disability. Data were collected 4 months before the start of the treatment (T - 4), at the start (T0), at the end (T4) and 4 months after the termination of the treatment (T + 4).
Results: Etravirine was reasonably tolerated, and adverse events were generally mild. Four months of etravirine treatment did not significantly increase the peak oxygen uptake but was associated with a change in the progression of the SARA score (p value < 0.001), compared to the 4 months pre- and post-treatment. It also significantly increased peak workload (p value = 0.021). No changes in the cardiac measures were observed. Health and QoL measures showed a worsening at the suspension of the drug.
Conclusions: In this open trial etravirine treatment was safe, reasonably well tolerated and appreciably improved neurological function and exercise performance. Even though a placebo effect cannot be ruled out, these results suggest that etravirine may represent a potential therapeutic agent in FRDA deserving testing in a randomized placebo-controlled clinical trial.
Keywords: Friedreich ataxia; efficacy; etravirine; safety; treatment.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Specifically, they do not own any intellectual property asset or rights, or any commercial interest of any kind, concerning any possible use of ETR in FRDA. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Campuzano V., Montermini L., Moltò M.D., Pianese L., Cossée M., Cavalcanti F., Monros E., Rodius F., Duclos F., Monticelli A., et al. Friedreich’s Ataxia: Autosomal Recessive Disease Caused by an Intronic GAA Triplet Repeat Expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. - DOI - PubMed
-
- Clark E., Johnson J., Dong Y.N., Mercado-Ayon E., Warren N., Zhai M., McMillan E., Salovin A., Lin H., Lynch D.R. Role of Frataxin Protein Deficiency and Metabolic Dysfunction in Friedreich Ataxia, an Autosomal Recessive Mitochondrial Disease. Neuronal Signal. 2018;2:NS20180060. doi: 10.1042/NS20180060. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

