Starvation and Inflammation Modulate Adipose Mesenchymal Stromal Cells' Molecular Signature
- PMID: 39202038
- PMCID: PMC11355917
- DOI: 10.3390/jpm14080847
Starvation and Inflammation Modulate Adipose Mesenchymal Stromal Cells' Molecular Signature
Abstract
Mesenchymal stromal cells (MSCs) and their released factors (secretome) are intriguing options for regenerative medicine approaches based on the management of inflammation and tissue restoration, as in joint disorders like osteoarthritis (OA). Production strategy may modulate cells and secretome fingerprints, and for the latter, the effect of serum removal by starvation used in clinical-grade protocols has been underestimated. In this work, the effect of starvation on the molecular profile of interleukin 1 beta (IL1β)-primed adipose-derived MSCs (ASCs) was tested by assessing the expression level of 84 genes related to secreted factors and 84 genes involved in defining stemness potential. After validation at the protein level, the effect of starvation modulation in the secretomes was tested in a model of OA chondrocytes. IL1β priming in vitro led to an increase in inflammatory mediators' release and reduced anti-inflammatory potential on chondrocytes, features reversed by subsequent starvation. Therefore, when applying serum removal-based clinical-grade protocols for ASCs' secretome production, the effects of starvation must be carefully considered and investigated.
Keywords: adipose mesenchymal stromal cells; chondrocytes; inflammation; osteoarthritis; secretome; starvation.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
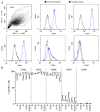
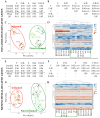
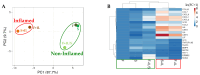

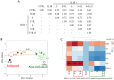
References
-
- Merimi M., El-Majzoub R., Lagneaux L., Moussa Agha D., Bouhtit F., Meuleman N., Fahmi H., Lewalle P., Fayyad-Kazan M., Najar M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021;9:661532. doi: 10.3389/fcell.2021.661532. - DOI - PMC - PubMed
-
- Miceli V., Zito G., Bulati M., Gallo A., Busà R., Iannolo G., Conaldi P.G. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J. Stem Cells. 2023;15:400–420. doi: 10.4252/wjsc.v15.i5.400. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

