AI-Driven Thoracic X-ray Diagnostics: Transformative Transfer Learning for Clinical Validation in Pulmonary Radiography
- PMID: 39202047
- PMCID: PMC11355475
- DOI: 10.3390/jpm14080856
AI-Driven Thoracic X-ray Diagnostics: Transformative Transfer Learning for Clinical Validation in Pulmonary Radiography
Abstract
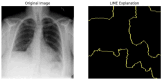
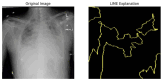
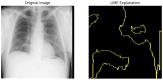
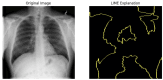
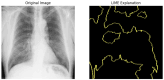
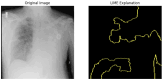
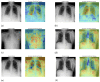
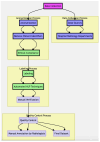
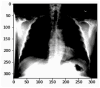
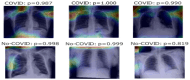
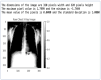
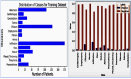
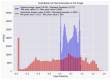
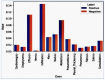
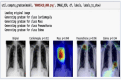
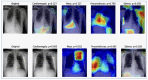
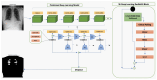
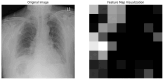
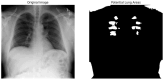
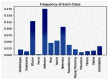
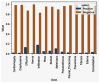
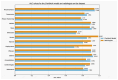
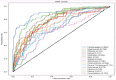
Our research evaluates advanced artificial (AI) methodologies to enhance diagnostic accuracy in pulmonary radiography. Utilizing DenseNet121 and ResNet50, we analyzed 108,948 chest X-ray images from 32,717 patients and DenseNet121 achieved an area under the curve (AUC) of 94% in identifying the conditions of pneumothorax and oedema. The model's performance surpassed that of expert radiologists, though further improvements are necessary for diagnosing complex conditions such as emphysema, effusion, and hernia. Clinical validation integrating Latent Dirichlet Allocation (LDA) and Named Entity Recognition (NER) demonstrated the potential of natural language processing (NLP) in clinical workflows. The NER system achieved a precision of 92% and a recall of 88%. Sentiment analysis using DistilBERT provided a nuanced understanding of clinical notes, which is essential for refining diagnostic decisions. XGBoost and SHapley Additive exPlanations (SHAP) enhanced feature extraction and model interpretability. Local Interpretable Model-agnostic Explanations (LIME) and occlusion sensitivity analysis further enriched transparency, enabling healthcare providers to trust AI predictions. These AI techniques reduced processing times by 60% and annotation errors by 75%, setting a new benchmark for efficiency in thoracic diagnostics. The research explored the transformative potential of AI in medical imaging, advancing traditional diagnostics and accelerating medical evaluations in clinical settings.
Keywords: artificial intelligence; deep learning; diagnostic accuracy; medical imaging; model interpretability; pulmonary radiography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
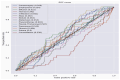
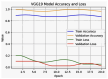


References
-
- Rajpurkar P., Irvin G., Ball R.L., Zhu K., Yang B., Mehta D., Lungren M.P., Ng A. Artificial intelligence for chest X-ray image analysis: A review. Lancet Digital Health. 2021;3:e67–e78.
-
- World Health Organization . Global Tuberculosis Report 2020. WHO; Geneva, Switzerland: 2020.
-
- Rajpurkar A., Irvin J., Zhu K., Yang B., Xu M., Wang H., Ng A.Y. Chexnet: Radiologist-level pneumonia detection on chest X-rays with deep learning. Nat. Med. 2017;23:1216–1222. doi: 10.48550/arXiv.1711.05225. - DOI
-
- Armato S.G., III, Giger M.L.., Macmahon H., Metz C.E., Nishikawa R.M., Doi K. Chest X-ray interpretation using deep learning: Challenges and opportunities. Radiology. 2019;290:105–115. doi: 10.1007/s40134-019-0333-9. - DOI
-
- Geron A. Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow. 2nd ed. O’Reilly Media. 2019. [(accessed on 31 July 2024)]. Available online: https://www.oreilly.com/library/view/hands-on-machine-learning/978149203....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

