Integrated Metagenomic and Metabolomics Profiling Reveals Key Gut Microbiota and Metabolites Associated with Weaning Stress in Piglets
- PMID: 39202331
- PMCID: PMC11354067
- DOI: 10.3390/genes15080970
Integrated Metagenomic and Metabolomics Profiling Reveals Key Gut Microbiota and Metabolites Associated with Weaning Stress in Piglets
Abstract
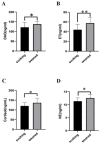
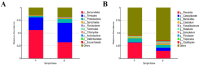
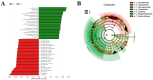
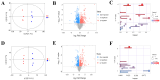
(1) Background: Weaning is a challenging and stressful event in the pig's life, which disrupts physiological balance and induces oxidative stress. Microbiota play a significant role during the weaning process in piglets. Therefore, this study aimed to investigate key gut microbiota and metabolites associated with weaning stress in piglets. (2) Methods: A total of ten newborn piglet littermates were randomly assigned to two groups: S (suckling normally) and W (weaned at 21 d; all euthanized at 23 d). Specimens of the cecum were dehydrated with ethanol, cleared with xylene, embedded in paraffin, and cut into 4 mm thick serial sections. After deparaffinization, the sections were stained with hematoxylin and eosin (H&E) for morphometric analysis. Cecal metagenomic and liver LC-MS-based metabolomics were employed in this study. Statistical comparisons were performed by a two-tailed Student's t-test, and p < 0.05 indicated statistical significance. (3) Results: The results showed that weaning led to intestinal morphological damage in piglets. The intestinal villi of suckling piglets were intact, closely arranged in an orderly manner, and finger-shaped, with clear contours of columnar epithelial cells. In contrast, the intestines of weaned piglets showed villous atrophy and shedding, as well as mucosal bleeding. Metagenomics and metabolomics analyses showed significant differences in composition and function between suckling and weaned piglets. The W piglets showed a decrease and increase in the relative abundance of Bacteroidetes and Proteobacteria (p < 0.05), respectively. The core cecal flora in W piglets were Campylobacter and Clostridium, while those in S piglets were Prevotella and Lactobacillus. At the phylum level, the relative abundance of Bacteroidetes significantly decreased (p < 0.05) in weaned piglets, while Proteobacteria significantly increased (p < 0.05). Significant inter-group differences were observed in pathways and glycoside hydrolases in databases, such as the KEGG and CAZymes, including fructose and mannose metabolism, salmonella infection, antifolate resistance, GH135, GH16, GH32, and GH84. We identified 757 differential metabolites between the groups through metabolomic analyses-350 upregulated and 407 downregulated (screened in positive ion mode). In negative ion mode, 541 differential metabolites were identified, with 270 upregulated and 271 downregulated. Major differential metabolites included glycerophospholipids, histidine, nitrogen metabolism, glycine, serine, threonine, β-alanine, and primary bile acid biosynthesis. The significant differences in glycine, serine, and threonine metabolites may be potentially related to dysbiosis caused by weaning stress. Taken together, the identification of microbiome and metabolome signatures of suckling and weaned piglets has paved the way for developing health-promoting nutritional strategies, focusing on enhancing bacterial metabolite production in early life stages.
Keywords: LC–MS/MS-based metabolomics; gut microbiota; metagenomic; piglet; weaning stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Grants and funding
- 202103a06020013/Major special science and technology project of Anhui Province
- GXXT-2023-059/the Cooperative Innovation Project of Anhui Provincial Universities
- 340000211260001000431/the Joint Research Project on Local Pig Breeding in Anhui Province
- 2021YFD1301200/National Key research and development Program of China
LinkOut - more resources
Full Text Sources

