Simulated Microgravity Alters Gene Regulation Linked to Immunity and Cardiovascular Disease
- PMID: 39202335
- PMCID: PMC11353732
- DOI: 10.3390/genes15080975
Simulated Microgravity Alters Gene Regulation Linked to Immunity and Cardiovascular Disease
Abstract
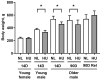
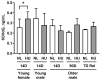
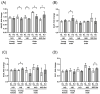
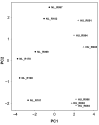
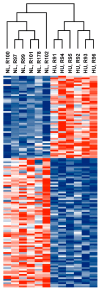
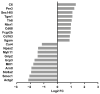
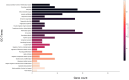
Microgravity exposure induces a cephalad fluid shift and an overall reduction in physical activity levels which can lead to cardiovascular deconditioning in the absence of countermeasures. Future spaceflight missions will expose crew to extended periods of microgravity among other stressors, the effects of which on cardiovascular health are not fully known. In this study, we determined cardiac responses to extended microgravity exposure using the rat hindlimb unloading (HU) model. We hypothesized that exposure to prolonged simulated microgravity and subsequent recovery would lead to increased oxidative damage and altered expression of genes involved in the oxidative response. To test this hypothesis, we examined hearts of male (three and nine months of age) and female (3 months of age) Long-Evans rats that underwent HU for various durations up to 90 days and reambulated up to 90 days post-HU. Results indicate sex-dependent changes in oxidative damage marker 8-hydroxydeoxyguanosine (8-OHdG) and antioxidant gene expression in left ventricular tissue. Three-month-old females displayed elevated 8-OHdG levels after 14 days of HU while age-matched males did not. In nine-month-old males, there were no differences in 8-OHdG levels between HU and normally loaded control males at any of the timepoints tested following HU. RNAseq analysis of left ventricular tissue from nine-month-old males after 14 days of HU revealed upregulation of pathways involved in pro-inflammatory signaling, immune cell activation and differential expression of genes associated with cardiovascular disease progression. Taken together, these findings provide a rationale for targeting antioxidant and immune pathways and that sex differences should be taken into account in the development of countermeasures to maintain cardiovascular health in space.
Keywords: cardiovascular system; hindlimb unloading; immune response; microgravity; transcriptomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Khine H.W., Steding-Ehrenborg K., Hastings J.L., Kowal J., Daniels J.D., Page R.L., Goldberger J.J., Ng J., Adams-Huet B., Bungo M.W., et al. Effects of Prolonged Spaceflight on Atrial Size, Atrial Electrophysiology, and Risk of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018;11:e005959. doi: 10.1161/CIRCEP.117.005959. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

