Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins
- PMID: 39202352
- PMCID: PMC11354059
- DOI: 10.3390/genes15080991
Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins
Abstract
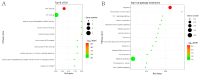
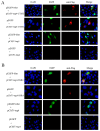
Atypical porcine pestivirus (APPV) can cause congenital tremor type A-II in neonatal piglets, posing a significant threat to swine herd health globally. Our previous study demonstrated that the Mut domains, comprising 112 amino acids at the N-terminus, are the primary functional regions of the E2 protein of APPV. This study identified 14 host cellular proteins that exhibit potential interactions with the Mut domains of the E2 protein using yeast two-hybrid screening. Using bioinformatics analysis, we discovered that the Mut domains of the E2 protein might exert regulatory effects on apoptosis by modulating energy metabolism within the mitochondria. We also conducted co-immunoprecipitation, glutathione S-transferase pull-down, and immunofluorescence assays to confirm the interaction between the Mut domains of the E2 protein and cathepsin H and signal sequence receptor subunit 4 (SSR4). Ultimately, SSR4 enhanced APPV replication in vitro. In summary, our study successfully elucidated the interactions between the Mut domains of the E2 protein and host cell protein, predicted the potential pathways implicated in these interactions, and demonstrated SSR4 involvement in APPV infection. These significant findings contribute valuable knowledge toward a deeper understanding of APPV pathogenesis and the role of the Mut domains of the E2 protein in this intricate process.
Keywords: APPV; E2 protein; Mut domains; SSR4; protein interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Characterization of the Humoral Immune Response Induced after Infection with Atypical Porcine Pestivirus (APPV).Viruses. 2019 Sep 21;11(10):880. doi: 10.3390/v11100880. Viruses. 2019. PMID: 31546571 Free PMC article.
-
Molecular characterization of two novel atypical porcine pestivirus (APPV) strains from piglets with congenital tremor in China.Transbound Emerg Dis. 2019 Jan;66(1):35-42. doi: 10.1111/tbed.13029. Epub 2018 Oct 22. Transbound Emerg Dis. 2019. PMID: 30281923
-
Evolution and genetic diversity of atypical porcine pestivirus (APPV) from piglets with congenital tremor in Guangxi Province, Southern China.Vet Med Sci. 2021 May;7(3):714-723. doi: 10.1002/vms3.407. Epub 2020 Dec 13. Vet Med Sci. 2021. PMID: 33314734 Free PMC article.
-
An emerging novel virus: Atypical porcine pestivirus (APPV).Rev Med Virol. 2019 Jan;29(1):e2018. doi: 10.1002/rmv.2018. Epub 2018 Nov 9. Rev Med Virol. 2019. PMID: 30411827 Review.
-
Pestivirus K (Atypical Porcine Pestivirus): Update on the Virus, Viral Infection, and the Association with Congenital Tremor in Newborn Piglets.Viruses. 2020 Aug 18;12(8):903. doi: 10.3390/v12080903. Viruses. 2020. PMID: 32824845 Free PMC article. Review.
References
-
- Postel A., Hansmann F., Baechlein C., Fischer N., Alawi M., Grundhoff A., Derking S., Tenhündfeld J., Pfankuche V.M., Herder V., et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016;6:27735. doi: 10.1038/srep27735. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

