A Closer Look into White Adipose Tissue Biology and the Molecular Regulation of Stem Cell Commitment and Differentiation
- PMID: 39202377
- PMCID: PMC11353785
- DOI: 10.3390/genes15081017
A Closer Look into White Adipose Tissue Biology and the Molecular Regulation of Stem Cell Commitment and Differentiation
Abstract
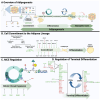
White adipose tissue (WAT) makes up about 20-25% of total body mass in healthy individuals and is crucial for regulating various metabolic processes, including energy metabolism, endocrine function, immunity, and reproduction. In adipose tissue research, "adipogenesis" is commonly used to refer to the process of adipocyte formation, spanning from stem cell commitment to the development of mature, functional adipocytes. Although, this term should encompass a wide range of processes beyond commitment and differentiation, to also include other stages of adipose tissue development such as hypertrophy, hyperplasia, angiogenesis, macrophage infiltration, polarization, etc.… collectively, referred to herein as the adipogenic cycle. The term "differentiation", conversely, should only be used to refer to the process by which committed stem cells progress through distinct phases of subsequent differentiation. Recognizing this distinction is essential for accurately interpreting research findings on the mechanisms and stages of adipose tissue development and function. In this review, we focus on the molecular regulation of white adipose tissue development, from commitment to terminal differentiation, and examine key functional aspects of WAT that are crucial for normal physiology and systemic metabolic homeostasis.
Keywords: adipogenesis; metabolism; mitotic clonal expansion; stem commitment and differentiation; white adipose tissue.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Martins T., Castro-Ribeiro C., Lemos S., Ferreira T., Nascimento-Gonçalves E., Rosa E., Oliveira P.A., Antunes L.M. Murine Models of Obesity. Obesities. 2022;2:127–147. doi: 10.3390/obesities2020012. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

