Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model
- PMID: 39202379
- PMCID: PMC11353984
- DOI: 10.3390/genes15081019
Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model
Abstract
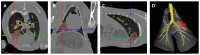
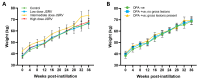
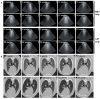
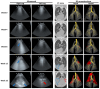
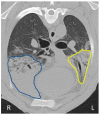
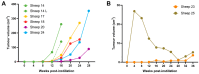
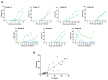
Ovine pulmonary adenocarcinoma (OPA) is an infectious, neoplastic lung disease of sheep that causes significant animal welfare and economic issues throughout the world. Understanding OPA pathogenesis is key to developing tools to control its impact. Central to this need is the availability of model systems that can monitor and track events after Jaagsiekte sheep retrovirus (JSRV) infection. Here, we report the development of an experimentally induced OPA model intended for this purpose. Using three different viral dose groups (low, intermediate and high), localised OPA tumour development was induced by bronchoscopic JSRV instillation into the segmental bronchus of the right cardiac lung lobe. Pre-clinical OPA diagnosis and tumour progression were monitored by monthly computed tomography (CT) imaging and trans-thoracic ultrasound scanning. Post mortem examination and immunohistochemistry confirmed OPA development in 89% of the JSRV-instilled animals. All three viral doses produced a range of OPA lesion types, including microscopic disease and gross tumours; however, larger lesions were more frequently identified in the low and intermediate viral groups. Overall, 31% of JSRV-infected sheep developed localised advanced lesions. Of the sheep that developed localised advanced lesions, tumour volume doubling times (calculated using thoracic CT 3D reconstructions) were 14.8 ± 2.1 days. The ability of ultrasound to track tumour development was compared against CT; the results indicated a strong significant association between paired CT and ultrasound measurements at each time point (R2 = 0.799, p < 0.0001). We believe that the range of OPA lesion types induced by this model replicates aspects of naturally occurring disease and will improve OPA research by providing novel insights into JSRV infectivity and OPA disease progression.
Keywords: animal models; computed tomography; ovine pulmonary adenocarcinoma; ultrasound.
Conflict of interest statement
The authors declare that C.C. and M.G. received a research grant from Johnson & Johnson to complete this study. J.S., N.S., and C.E.E. are employees of Johnson & Johnson. The funders had no role in the collection, analysis, or interpretation of data or in the decision to publish the results. C.E.E. made critical revisions to the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fan H. Jaagsiekte Sheep Retrovirus and Lung Cancer. Volume 275 Springer Science & Business Media; Berlin/Heidelberg, Germany: 2003.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

