ARGONAUTE2 Localizes to Sites of Sporocysts in the Schistosome-Infected Snail, Biomphalaria glabrata
- PMID: 39202383
- PMCID: PMC11353429
- DOI: 10.3390/genes15081023
ARGONAUTE2 Localizes to Sites of Sporocysts in the Schistosome-Infected Snail, Biomphalaria glabrata
Abstract
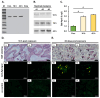
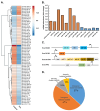
MicroRNAs (miRNAs) are a class of small regulatory RNA that are generated via core protein machinery. The miRNAs direct gene-silencing mechanisms to mediate an essential role in gene expression regulation. In mollusks, miRNAs have been demonstrated to be required to regulate gene expression in various biological processes, including normal development, immune responses, reproduction, and stress adaptation. In this study, we aimed to establishment the requirement of the miRNA pathway as part of the molecular response of exposure of Biomphalaria glabrata (snail host) to Schistosoma mansoni (trematode parasite). Initially, the core pieces of miRNA pathway protein machinery, i.e., Drosha, DGCR8, Exportin-5, Ran, and Dicer, together with the central RNA-induced silencing complex (RISC) effector protein Argonaute2 (Ago2) were elucidated from the B. glabrata genome. Following exposure of B. glabrata to S. mansoni miracidia, we identified significant expression up-regulation of all identified pieces of miRNA pathway protein machinery, except for Exportin-5, at 16 h post exposure. For Ago2, we went on to show that the Bgl-Ago2 protein was localized to regions surrounding the sporocysts in the digestive gland of infected snails 20 days post parasite exposure. In addition to documenting elevated miRNA pathway protein machinery expression at the early post-exposure time point, a total of 13 known B. glabrata miRNAs were significantly differentially expressed. Of these thirteen B. glabrata miRNAs responsive to S. mansoni miracidia exposure, five were significantly reduced in their abundance, and correspondingly, these five miRNAs were determined to putatively target six genes with significantly elevated expression and that have been previously associated with immune responses in other animal species, including humans. In conclusion, this study demonstrates the central importance of a functional miRNA pathway in snails, which potentially forms a critical component of the immune response of snails to parasite exposure. Further, the data reported in this study provide additional evidence of the complexity of the molecular response of B. glabrata to S. mansoni infection: a molecular response that could be targeted in the future to overcome parasite infection and, in turn, human schistosomiasis.
Keywords: Argonaute2 (Ago2); Biomphalaria glabrata; Schistosoma mansoni; host–parasite interaction; immune response; miRNA pathway protein machinery; miRNA-directed gene expression regulation; microRNA (miRNA) pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

