Activation of the CDK7 Gene, Coding for the Catalytic Subunit of the Cyclin-Dependent Kinase (CDK)-Activating Kinase (CAK) and General Transcription Factor II H, by the Trans-Activator Protein Tax of Human T-Cell Leukemia Virus Type-1
- PMID: 39202439
- PMCID: PMC11353830
- DOI: 10.3390/genes15081080
Activation of the CDK7 Gene, Coding for the Catalytic Subunit of the Cyclin-Dependent Kinase (CDK)-Activating Kinase (CAK) and General Transcription Factor II H, by the Trans-Activator Protein Tax of Human T-Cell Leukemia Virus Type-1
Abstract
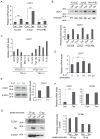
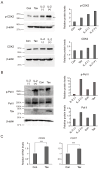
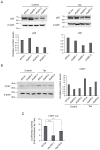
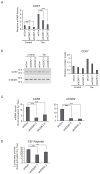
Human T-cell leukemia virus type-1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL). The trans-activator protein Tax of HTLV-1 plays crucial roles in leukemogenesis by promoting proliferation of virus-infected cells through activation of growth-promoting genes. However, critical target genes are yet to be elucidated. We show here that Tax activates the gene coding for cyclin-dependent kinase 7 (CDK7), the essential component of both CDK-activating kinase (CAK) and general transcription factor TFIIH. CAK and TFIIH play essential roles in cell cycle progression and transcription by activating CDKs and facilitating transcriptional initiation, respectively. Tax induced CDK7 gene expression not only in human T-cell lines but also in normal peripheral blood lymphocytes (PHA-PBLs) along with increased protein expression. Tax stimulated phosphorylation of CDK2 and RNA polymerase II at sites reported to be mediated by CDK7. Tax activated the CDK7 promoter through the NF-κB pathway, which mainly mediates cell growth promotion by Tax. Knockdown of CDK7 expression reduced Tax-mediated induction of target gene expression and cell cycle progression. These results suggest that the CDK7 gene is a crucial target of Tax-mediated trans-activation to promote cell proliferation by activating CDKs and transcription.
Keywords: ATL; CDK7; HTLV-1; Tax; trans-activation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I.Oncogene. 2001 Apr 19;20(17):2055-67. doi: 10.1038/sj.onc.1204304. Oncogene. 2001. PMID: 11360190
-
Trans-Activation of the Coactivator-Associated Arginine Methyltransferase 1 (Carm1) Gene by the Oncogene Product Tax of Human T-Cell Leukemia Virus Type 1.Genes (Basel). 2024 May 27;15(6):698. doi: 10.3390/genes15060698. Genes (Basel). 2024. PMID: 38927636 Free PMC article.
-
CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point.PLoS Genet. 2013 May;9(5):e1003546. doi: 10.1371/journal.pgen.1003546. Epub 2013 May 30. PLoS Genet. 2013. PMID: 23737759 Free PMC article.
-
CAK-Cyclin-dependent Activating Kinase: a key kinase in cell cycle control and a target for drugs?Cell Cycle. 2005 Apr;4(4):572-7. Epub 2005 Apr 16. Cell Cycle. 2005. PMID: 15876871 Review.
-
CDK7 inhibitors as anticancer drugs.Cancer Metastasis Rev. 2020 Sep;39(3):805-823. doi: 10.1007/s10555-020-09885-8. Cancer Metastasis Rev. 2020. PMID: 32385714 Free PMC article. Review.
References
-
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K.I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

