Genetic and Pathophysiological Basis of Cardiac and Skeletal Muscle Laminopathies
- PMID: 39202453
- PMCID: PMC11354015
- DOI: 10.3390/genes15081095
Genetic and Pathophysiological Basis of Cardiac and Skeletal Muscle Laminopathies
Abstract
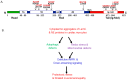
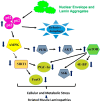
Nuclear lamins, a type V intermediate filament, are crucial components of the nuclear envelope's inner layer, maintaining nuclear integrity and mediating interactions between the nucleus and cytoplasm. Research on human iPSC-derived cells and animal models has demonstrated the importance of lamins in cardiac and skeletal muscle development and function. Mutations in lamins result in laminopathies, a group of diseases including muscular dystrophies, Hutchison-Gilford progeria syndrome, and cardiomyopathies with conduction defects. These conditions have been linked to disrupted autophagy, mTOR, Nrf2-Keap, and proteostasis signaling pathways, indicating complex interactions between the nucleus and cytoplasm. Despite progress in understanding these pathways, many questions remain about the mechanisms driving lamin-induced pathologies, leading to limited therapeutic options. This review examines the current literature on dysregulated pathways in cardiac and skeletal muscle laminopathies and explores potential therapeutic strategies for these conditions.
Keywords: Nrf2-signaling; aging; autophagy-signaling; cardiomyopathy and skeletal muscle dysfunction; laminopathies; redox-homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

