Synthesis, Structural Properties and Biological Activities of Novel Hydrazones of 2-, 3-, 4-Iodobenzoic Acid
- PMID: 39202893
- PMCID: PMC11356900
- DOI: 10.3390/molecules29163814
Synthesis, Structural Properties and Biological Activities of Novel Hydrazones of 2-, 3-, 4-Iodobenzoic Acid
Abstract
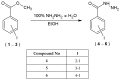
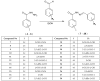
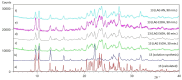
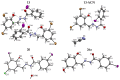
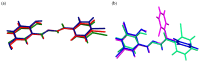
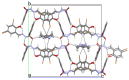
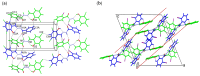
Nowadays, searching for novel antimicrobial agents is crucial due to the increasing number of resistant bacterial strains. Moreover, cancer therapy is a major challenge for modern medicine. Currently used cytostatics have a large number of side effects and insufficient therapeutic effects. Due to the above-mentioned facts, we undertook research to synthesize novel compounds from the acylhydrazone group aimed at obtaining potential antimicrobial and anticancer agents. As a starting material, we employed hydrazides of 2-, 3- or 4-iodobenzoic acid, which gave three series of acylhydrazones in the condensation reaction with various aldehydes. The chemical structure of all obtained compounds was confirmed by IR, 1H NMR, and 13C NMR. The structure of selected compounds was determined by single-crystal X-ray diffraction analysis. Additionally, all samples were characterized using powder X-ray diffraction. The other issue in this research was to examine the possibility of the solvent-free synthesis of compounds using mechanochemical methods. The biological screening results revealed that some of the newly synthesized compounds indicated a beneficial antimicrobial effect even against MRSA-the methicillin-resistant Staphylococcus aureus ATCC 43300 strain. In many cases, the antibacterial activity of synthesized acylhydrazones was equal to or better than that of commercially available antibacterial agents that were used as reference substances in this research. Significantly, the tested compounds do not show toxicity to normal cell lines either.
Keywords: acylhydrazones; antimicrobial activity; bioactivity; crystal structure; cytotoxicity; iodobenzoic acid derivatives.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Synthesis and antibacterial evaluation of New N-acylhydrazone derivatives from dehydroabietic acid.Molecules. 2012 Apr 20;17(4):4634-50. doi: 10.3390/molecules17044634. Molecules. 2012. PMID: 22522394 Free PMC article.
-
Synthesis and in vitro bioactivity study of new hydrazide-hydrazones of 5-bromo-2-iodobenzoic acid.Biomed Pharmacother. 2020 Oct;130:110526. doi: 10.1016/j.biopha.2020.110526. Epub 2020 Jul 18. Biomed Pharmacother. 2020. PMID: 32693180
-
Searching for novel antimicrobial agents among hydrazide-hydrazones of 4-iodosalicylic acid.Biomed Pharmacother. 2022 Sep;153:113302. doi: 10.1016/j.biopha.2022.113302. Epub 2022 Jun 17. Biomed Pharmacother. 2022. PMID: 35724512
-
Transition metal complexes of hydrazones as potential antimicrobial and anticancer agents: A short review.Chem Biol Drug Des. 2024 Jul;104(1):e14590. doi: 10.1111/cbdd.14590. Chem Biol Drug Des. 2024. PMID: 39039615 Review.
-
Trends in the Synthesis of Antimicrobial Derivatives by using the Gewald, Strecker, and Groebke-Blackburn-Bienaymé (GBB) Reactions.Med Chem. 2024;20(7):663-688. doi: 10.2174/0115734064282699240315042428. Med Chem. 2024. PMID: 38523542 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

