Tuning Ferulic Acid Solubility in Choline-Chloride- and Betaine-Based Deep Eutectic Solvents: Experimental Determination and Machine Learning Modeling
- PMID: 39202918
- PMCID: PMC11357058
- DOI: 10.3390/molecules29163841
Tuning Ferulic Acid Solubility in Choline-Chloride- and Betaine-Based Deep Eutectic Solvents: Experimental Determination and Machine Learning Modeling
Abstract
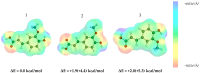
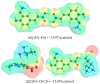
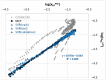
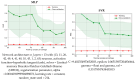
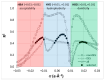
Deep eutectic solvents (DES) represent a promising class of green solvents, offering particular utility in the extraction and development of new formulations of natural compounds such as ferulic acid (FA). The experimental phase of the study undertook a systematic investigation of the solubility of FA in DES, comprising choline chloride or betaine as hydrogen bond acceptors and six different polyols as hydrogen bond donors. The results demonstrated that solvents based on choline chloride were more effective than those based on betaine. The optimal ratio of hydrogen bond acceptors to donors was found to be 1:2 molar. The addition of water to the DES resulted in a notable enhancement in the solubility of FA. Among the polyols tested, triethylene glycol was the most effective. Hence, DES composed of choline chloride and triethylene glycol (TEG) (1:2) with added water in a 0.3 molar ration is suggested as an efficient alternative to traditional organic solvents like DMSO. In the second part of this report, the affinities of FA in saturated solutions were computed for solute-solute and all solute-solvent pairs. It was found that self-association of FA leads to a cyclic structure of the C28 type, common among carboxylic acids, which is the strongest type of FA affinity. On the other hand, among all hetero-molecular bi-complexes, the most stable is the FA-TEG pair, which is an interesting congruency with the high solubility of FA in TEG containing liquids. Finally, this work combined COSMO-RS modeling with machine learning for the development of a model predicting ferulic acid solubility in a wide range of solvents, including not only DES but also classical neat and binary mixtures. A machine learning protocol developed a highly accurate model for predicting FA solubility, significantly outperforming the COSMO-RS approach. Based on the obtained results, it is recommended to use the support vector regressor (SVR) for screening new dissolution media as it is not only accurate but also has sound generalization to new systems.
Keywords: COSMO-RS; deep eutectic solvents; ferulic acid; green solvents; machine learning; molecular interactions; solubility; support vector regressor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


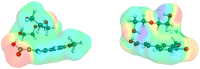
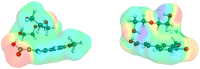

References
-
- Ou S., Kwok K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269. doi: 10.1002/jsfa.1873. - DOI
LinkOut - more resources
Full Text Sources

